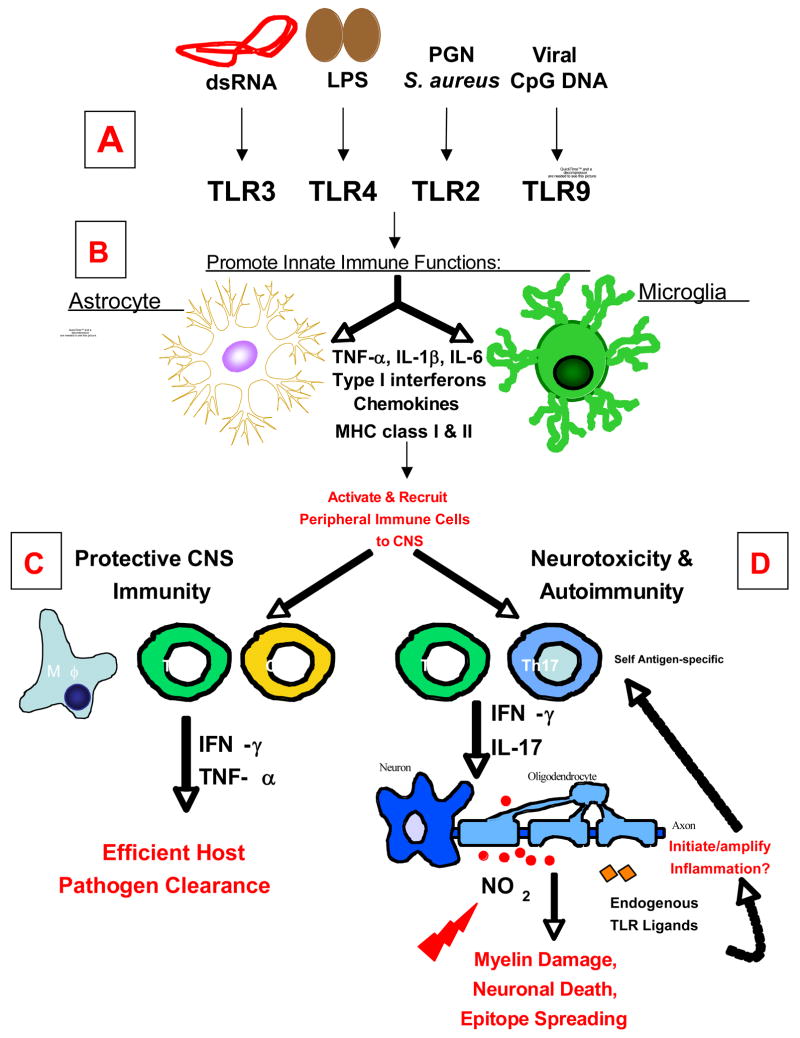
Figure 1. Schematic Representation of the Roles of TLR Signaling on Glial Cells in CNS Immunity and Disease.
Glial cells, astrocytes and microglia, express a wide variety of TLRs. (A) Stimulation with TLR ligands: dsRNA (TLR3), LPS (TLR4), peptidoglycans (PGN; TLR2 with TLR1/6), and viral CpG DNA (TLR9) promotes an array of immune functions in glial cells, including, the secretion of pro-inflammatory cytokines, chemokines, type I interferons (IFN-α/β), and an increase in MHC class I and II expression. (B) Secretion of these molecules affects the adaptive immune responses in the CNS by promoting a pro-inflammatory response thereby recruiting leukocytes (macrophages, dendritic cells, and CD4+ and CD8+ T cells) to the CNS. (C) Together these functions are essential for initiating protective immunity and efficient clearance of invading pathogens from the CNS. (D) However recent evidence suggests that depending on the type of pathogen and/or route of infection, chronic activation of TLRs on glial cells may be detrimental to the host, causing neurotoxicity and oligodendrocyte or neuronal cell death. In addition to the role of TLR signaling on glial cells in protective immunity, it may also be important for the development of CNS autoimmune diseases in the absence of pathogens. The CNS inflammatory response triggered by a virus or injury leads to further CNS tissue damage. The release of sequestered myelin epitopes further propagates inflammation and self-antigen specific immune responses (Th1 and Th17 responses), thus initiating autoimmunity. TLRs on glial cells can also be activated by endogenous ligands released during tissue damage that may initiate and/or further amplify inflammation in CNS autoimmune disease.