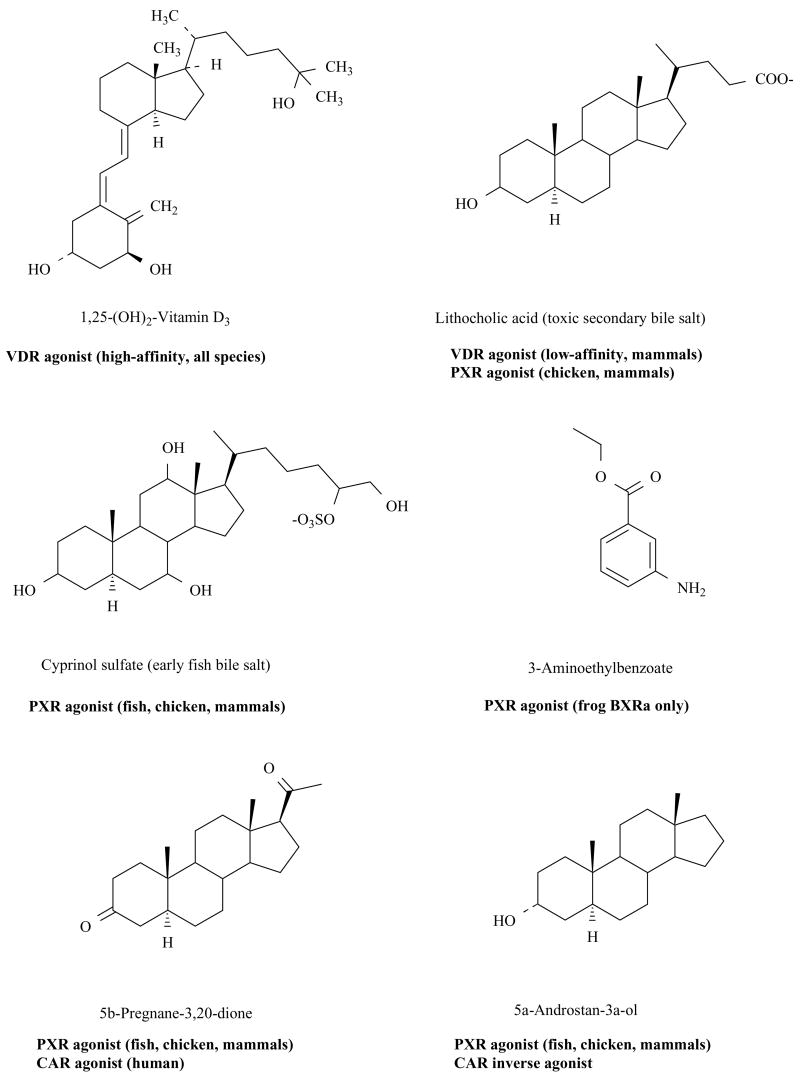
Fig. (1). Endogenous Ligands of VDR, PXR, and CAR.
Chemical structures of endogenous ligands for VDR, PXR, and CAR are shown, indicating compounds that overlap between receptors. 1,25-(OH)2-Vitamin D3 (calcitriol) is a selective high-affinity agonist for all VDRs and shows no activity at CARs or PXRs. Lithocholic acid, a potentially toxic evolutionarily ‘recent’ bile acid, activates mammalian VDRs and mammalian/chicken PXRs. VDRs and PXRs appear to have recently evolved to respond to LCA. Cyprinol sulfate, a 5α- C27 bile alcohol sulfate representative of the earliest bile salts to evolve in vertebrates, activates all PXRs except for the frog BXRs. 3-Aminoethylbenzoate, a compound isolated from Xenopus laevis and shown to have a role in Xenopus development, is fairly selective for Xenopus BXRα, especially in terms of the efficacy of its effect. Pregnane and androstane ligands overlap between CARs and PXRs with some steroids such as 5α-androstan-3α-ol showing strong inverse agonist actions at mouse CAR.