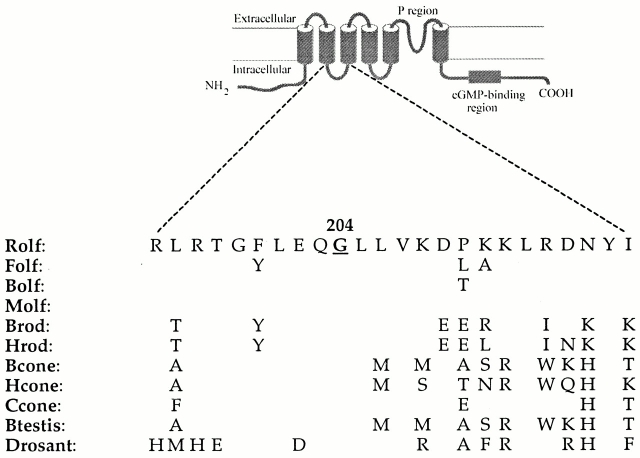
Figure 1.
Amino acid sequences of the S2–S3 loop of various wild-type CNG channels. Top diagram demonstrates the primary structure and membrane topology of a CNG channel. All wild-type sequences contain a glycine residue at the site equivalent to position 204 (highlighted in bold and underlined) in the Rolf channel. Residues corresponding to the equivalent Rolf residues at positions 199–207 are very highly conserved in all channels. In contrast, there is more variation in the carboxyl-terminal half of the loop sequence. Rolf, rat olfactory; Folf, fish olfactory; Bolf, bovine olfactory; Molf, mouse olfactory; Brod, bovine rod; Hrod, human rod; Bcone, bovine cone; Hcone, human cone; Ccone, chick cone; Btestis, bovine testis; and Drosant, Drosophila antenna. The original Molf clone contained a mutation at position 204 (Molf G204E), and we mutated the glutamate (E) to the wild-type glycine (G). For the Rolf channel, the glycine at 204 was replaced with either glutamate, aspartate (D), or tryptophan (W). Replacement with a lysine residue (K) gave no functional expression.