Figure 7.
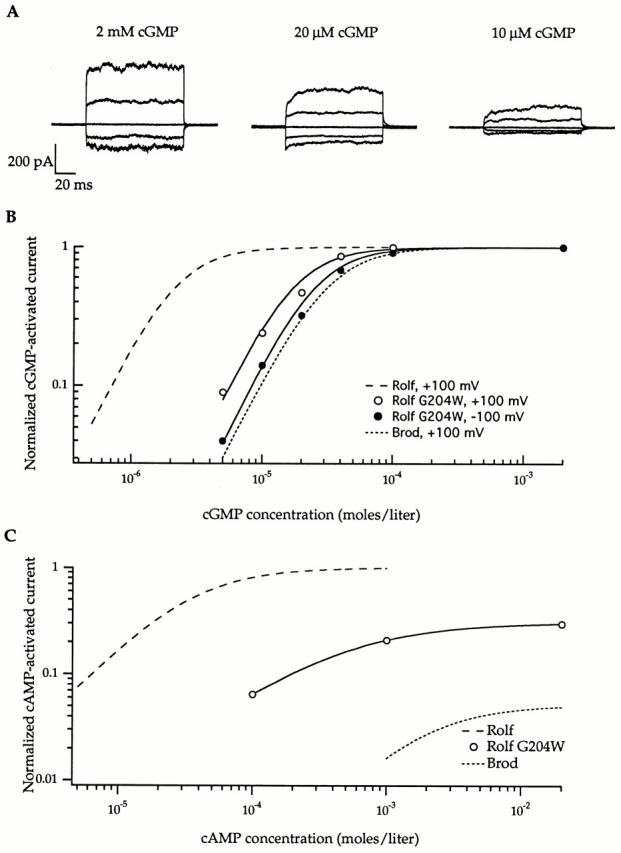
Introduction of tryptophan at position 204 produced a mutant Rolf channel with measurable gating kinetics, lower apparent agonist affinity, and decreased cAMP efficacy. (A) The current families were obtained with the designated amounts of cGMP in response to voltage jumps ranging from −100 to +100 mV in 50-mV steps, from a holding potential of 0 mV. The traces were corrected for leak by subtracting responses in the absence of cGMP. (B) The dose–response curves for activation by cGMP at + 100 mV and −100 mV are shown for a single patch containing the Rolf G204W mutant; the data were fit with the Hill relation, as in Fig. 2 (see legend). For this patch, at +100 mV, the cGMP K1/2 = 14 μM and n = 2.0; and at −100 mV, the cGMP K1/2 = 18 μM and n = 2.0. For averaged data from six patches, at +100 mV, the K1/2 = 14 μM and n = 1.7; and at −100 mV, the K1/2 = 19 μM and n = 1.7. The Hill fits (Fig. 2) for the cGMP dose–response curves of the Rolf and Brod channels (at +100 mV) are shown for comparison. (C) cAMP is only a partial agonist for Rolf G204W, unlike the Rolf channel (Hill fit from Fig. 2 is shown for comparison). A dose–response curve for activation by cAMP is shown for a single patch: K1/2 = 440 μM and n = 0.9. The average efficacy for two patches was 44%.
