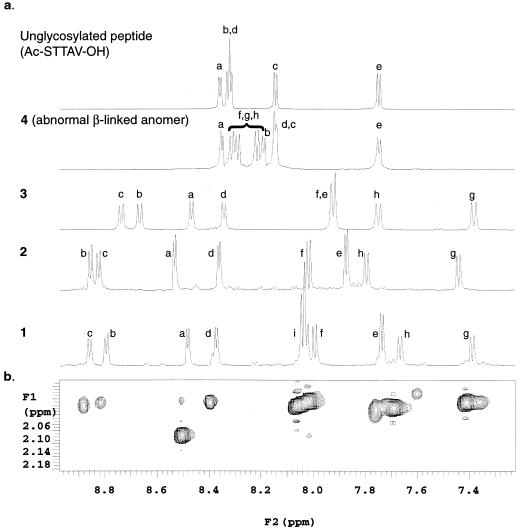
Figure 2.
One- and two-dimensional NMR analysis∥ demonstrates that sequential O-glycosylation of α-linked GalNAc induces transition to a highly stable structure. (a) The amide region of the 1H-NMR for the peptide Ac-STTAV-OH, the β-linked 4, and the α-linked series 3, 2, and 1. The amide proton resonances of the peptide backbone, STTAV, are labeled a, b, c, d, and e, respectively. The GalNAc amide resonances on the STT segments of 1–4 are labeled f, g, and h, respectively, and the sialic acid amide signals are labeled i. Comparison of these spectra shows that NH patterns in abnormal (β-linked) anomer 4 differ little from the unglycosylated peptide in contrast to the spectra of α-linked clusters, which reveal dramatic dispersion of resonances. (b) Two-dimensional NOESY measurements reveal strong interactions between the methyl protons of the GalNAc acetyl groups and the peptide amide protons for 1. Similar interactions were observed for α-linked 2 and 3. No such interactions were observed in the β-linked 4.