Fig. 3.
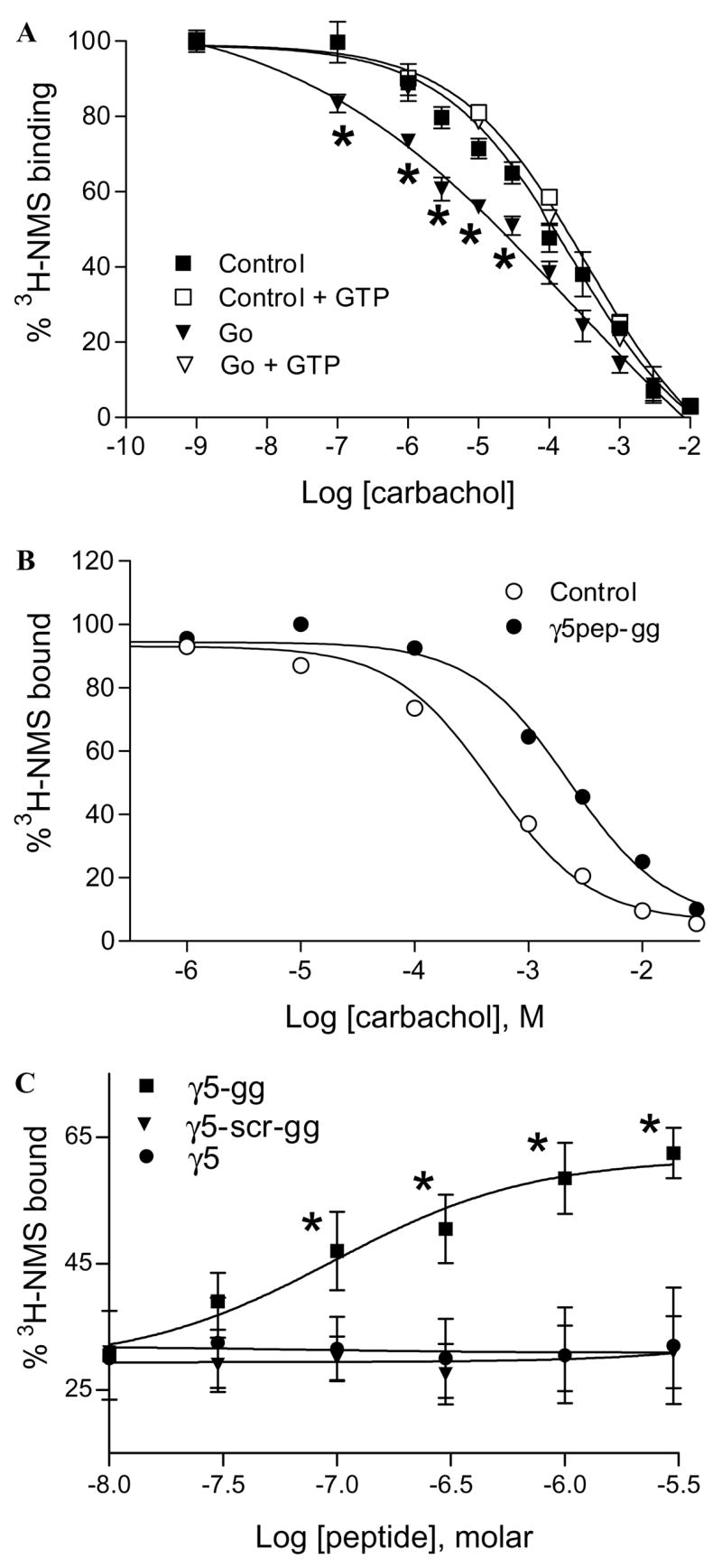
Effects of G protein and γ5 peptides on M2 affinity for carbachol. (A) G protein increases the affinity of M2 for carbachol. M2 in membrane suspension was incubated with or without the Go heterotrimer as described under Materials and methods. Samples were diluted and incubated with [3H]NMS and carbachol. Some samples were treated with 100 μM GTPγS to dissociate the G protein/receptor complexes. Samples were washed on glass filters and radioactivity was counted as described earlier. Non-specific binding was measured in the presence of 10 μM atropine. Points represent mean ± SEM (n ≥ 5). Asterisks denote that the [3H]NMS binding to the M2–Go complex is significantly lower (p < 0.05) compared to the free receptor. Data points were curve-fitted by non-linear regression using GraphPad Prism. (B) γ5pep-gg affects M2 affinity for carbachol. M2 receptors in membranes were preincubated with γ5 peptides. Samples after dilution were incubated with [3H]NMS and carbachol as described before. The final concentrations were 5 nM M2, 10 μM sodium cholate, 1 μM γ5pep-gg, 10 nM [3H]NMS, and increasing concentrations of carbachol. Representative result is shown from at least four independent experiments in duplicate. Data points were curve-fitted as described before. (C) Dose dependence of M2 receptor affinity for carbachol in the presence of γ5 peptides. The effects of peptides specific to γ5 on M2 receptor affinity for carbachol were measured as in (B). Increasing concentrations of peptides were added at 1 mM constant concentration of carbachol. Points represent means ± SEM (n ≥ 3). Asterisks denote that the M2 receptors bind significantly higher concentrations of [3H]NMS (p < 0.05) in the presence of the wild type peptide compared to the mutant peptide. The dose–response curves were generated by non-linear regression using Prism.
