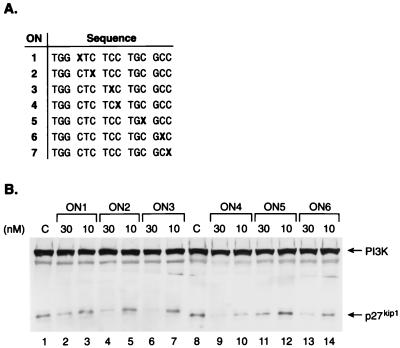
Figure 2.
The bioactivity of the G-clamp-modified p27kip1 S-ON is context-dependent. (A) Sequence and position of the G-clamp modification (G-clamp is denoted by X) tested for p27kip1 antisense activity. All linkages are phosphorothioate. T, thymidine; G, 2′-deoxyguanosine; C, 2′-deoxy-5-methylcytidine. (B) Immunodetection of p27kip1 and PI3K (p85 regulatory subunit) after transfection of CV-1 cells with GS3815 cytofectin alone (2.5 μg/ml; lane 1), or GS3815 cytofectin (2.5 μg/ml) complexed with 30 or 10 nM of ON1 (lanes 2 and 3), ON2 (lanes 4 and 5), ON3 (lanes 6 and 7), ON4 (lanes 9 and 10), ON5 (lanes 11 and 12), or ON6 (lanes 13 and 14). At 30 nM, ON1 inhibited p27kip1 protein levels by 40%, ON2 by 72%, ON3 by 88%, ON4 by 98%, ON5 by 54%, and ON6 by 98%. No inhibition of p27kip1 protein levels was observed for ON1, ON2, ON3, and ON5 at 10 nM. The two most potent S-ONs, ON4 and ON6, inhibited p27kip1 protein levels by 84 and 57%, respectively, at 10 nM (lanes 10 and 14). PI3K was used as an internal control for protein integrity, concentration, and transfer variations among the samples.