Figure 6.
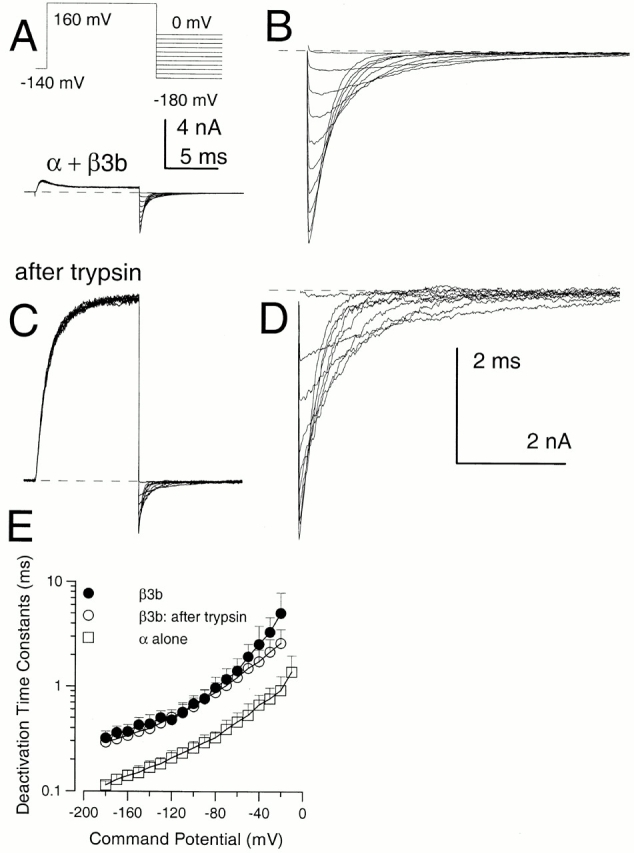
Effects of trypsin on α + β3b currents. In A, currents in an inside-out patch expressing α + β3b channels were activated by the indicated voltage protocol with 10 μM Ca2+. In B, the tail currents at potentials from 0 mV to −180 mV are displayed at higher magnification. In C, trypsin was briefly applied to the same patch shown in A resulting in removal of inactivation, whereas D shows the tail currents after trypsin at higher magnification. After trypsin, peak outward current at +160 mV appears to activate more slowly and is markedly larger. In contrast, tail current amplitudes exhibit a more modest increase after trypsin. The calibration bar in A also applies to C, while B and D share a calibration bar. Tail current amplitude at more positive repolarization potentials exhibits a larger increase following trypsin than those at more negative potentials. After trypsin application, the tail current decay follows a relatively simple exponential time course, whereas, before trypsin, there is a brief rising phase. In E, time constants of deactivation (τd) are plotted for α + β3b currents before (•) and after (○) trypsin and compared with τd for currents arising from α alone (□). Tail currents for intact α + β3b were fit with a two exponential function to approximate the fast unblocking shoulder of current and then the deactivation of current. Points show means and standard deviations for at least four patches.
