Abstract
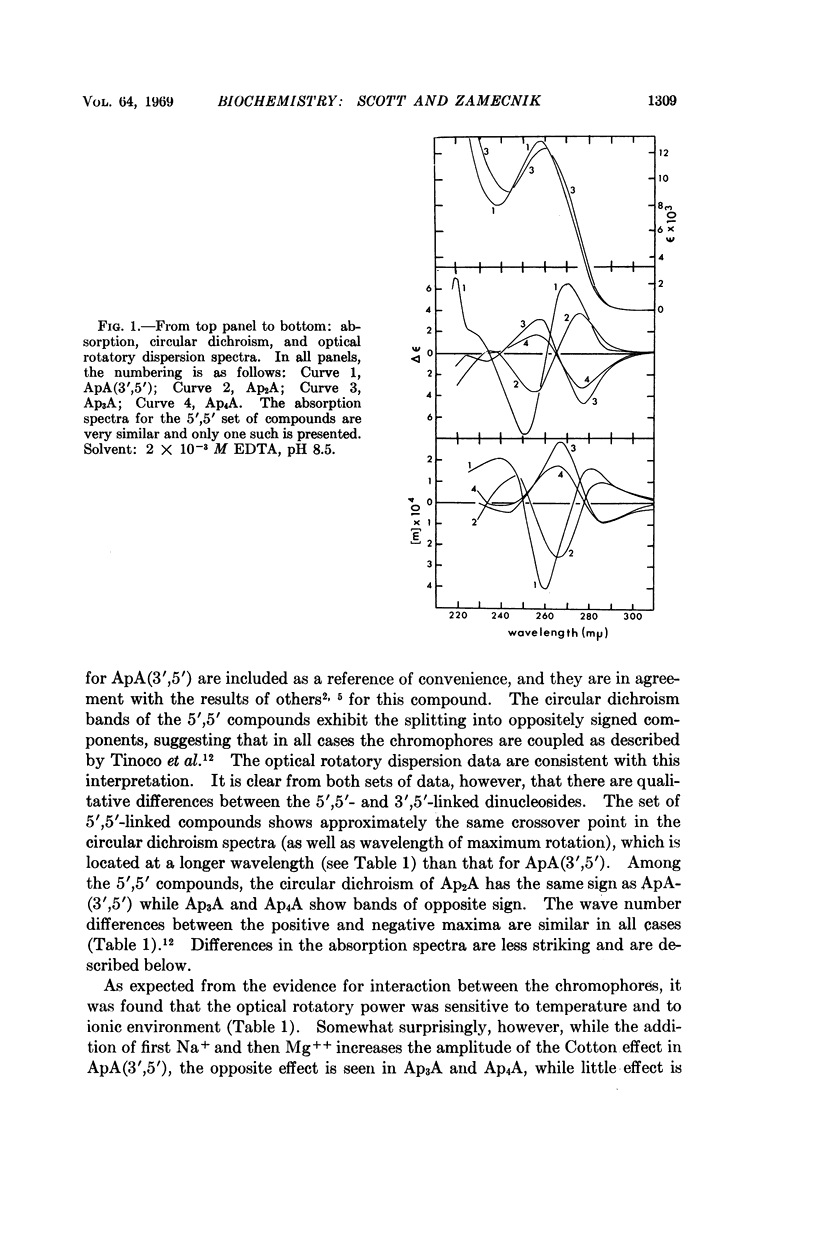
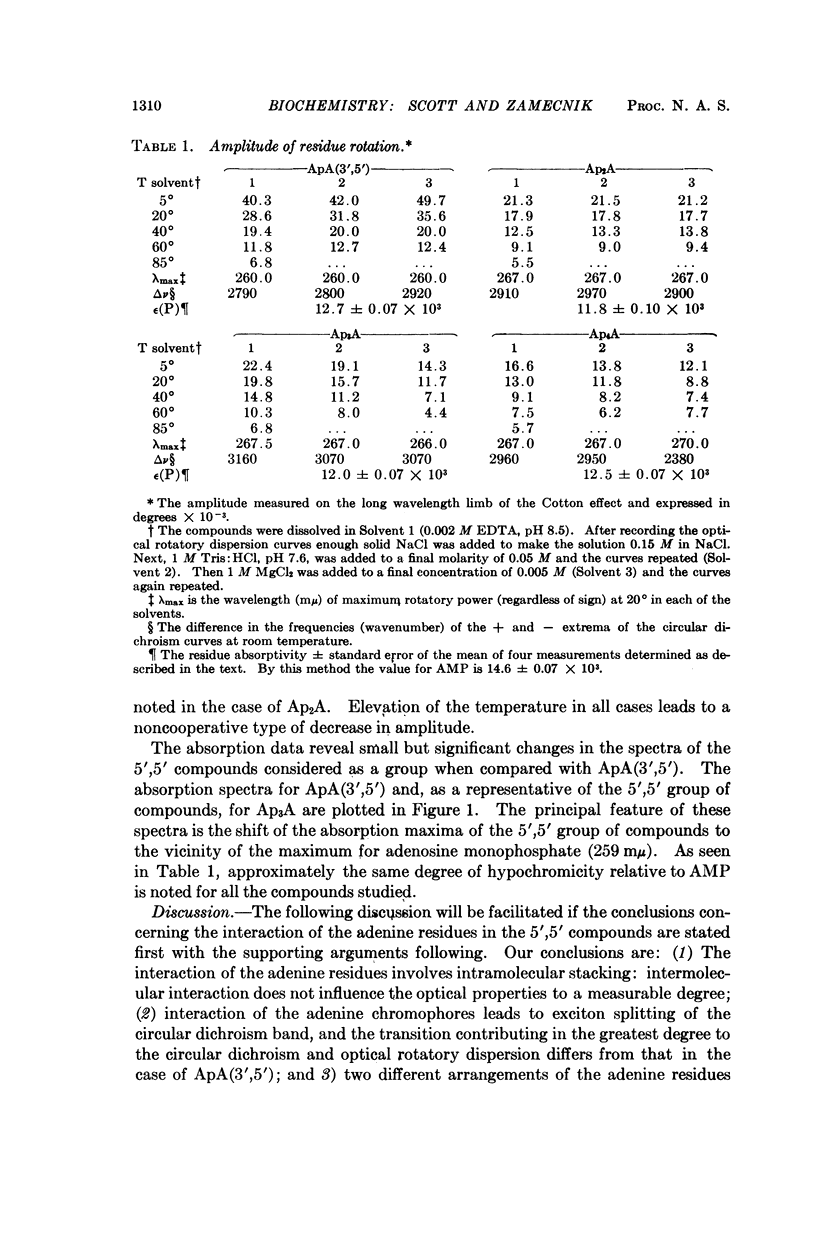
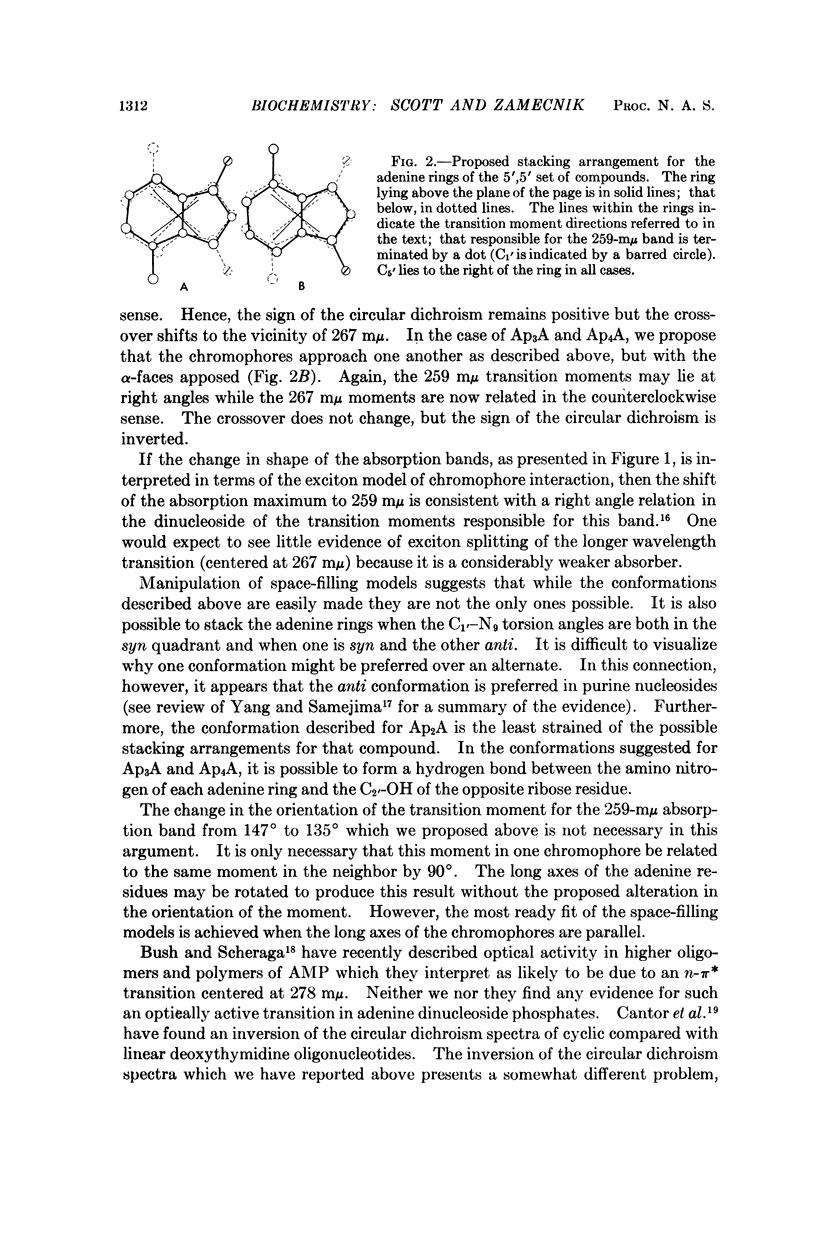
The absorption spectra, optical rotatory dispersion, and circular dichroism of a series of diadenosine-5′-phosphates, differing in the length of the phosphate bridge, indicate that in this set of compounds the adenine residues form an intramolecular stacked conformation. The differences in the optical properties suggest that this arrangement is different from that deduced by others for diadenosine-3′,5′-monophosphate (ApA(3′,5′)) but is of comparable stability.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahms J., Michelson A. M., Van Holde K. E. Adenylate oligomers in single- and double-strand conformation. J Mol Biol. 1966 Feb;15(2):467–488. doi: 10.1016/s0022-2836(66)80122-5. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Fairclough R. H., Newmark R. A. Oligonucleotide interactions. II. Differences in base stacking in linear and cyclic deoxythymidine oligonucleotides. Biochemistry. 1969 Sep;8(9):3610–3617. doi: 10.1021/bi00837a018. [DOI] [PubMed] [Google Scholar]
- DEVOE H., TINOCO I., Jr The hypochromism of helical polynucleotides. J Mol Biol. 1962 Jun;4:518–527. doi: 10.1016/s0022-2836(62)80106-5. [DOI] [PubMed] [Google Scholar]
- MASSOULIE J., MICHELSON M. POLYNUCL'EOTIDES. PROPRI'ET'ES PHYSIQUES DE DINUCL'EOTIDES. C R Hebd Seances Acad Sci. 1964 Oct 28;259:2923–2926. [PubMed] [Google Scholar]
- Michelson A. M., Ulbricht T. L., Emerson T. R., Swan R. J. Optical rotatory dispersion of oligoadenylic acids and a consideration of the factors stabilizing helical polynucleotides. Nature. 1966 Feb 26;209(5026):873–874. doi: 10.1038/209873a0. [DOI] [PubMed] [Google Scholar]
- Miles D. W., Robins M. J., Robins R. K., Eyring H. Circular dichroism of nucleoside derivatives. VI. The optically active bands of adenine nucleoside derivatives. Proc Natl Acad Sci U S A. 1969 Jan;62(1):22–29. doi: 10.1073/pnas.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman B., Pullman A. Quantum-mechanical investigations of the electronic structure of nucleic acids and their constituents. Prog Nucleic Acid Res Mol Biol. 1969;9:327–402. doi: 10.1016/s0079-6603(08)60772-2. [DOI] [PubMed] [Google Scholar]
- RICH A., DAVIES D. R., CRICK F. H., WATSON J. D. The molecular structure of polyadenylic acid. J Mol Biol. 1961 Feb;3:71–86. doi: 10.1016/s0022-2836(61)80009-0. [DOI] [PubMed] [Google Scholar]
- Randerath K., Janeway C. M., Stephenson M. L., Zamecnik P. C. Isolation and characterization of dinucleoside tetra- and tri-phosphates formed in the presence of lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):98–105. doi: 10.1016/0006-291x(66)90416-5. [DOI] [PubMed] [Google Scholar]
- Seno T., Kobayashi M., Nishimura S. Characteristic behavior of 4-thiouridine region of individual amino acid-specific Escherichia coli tRNA's upon heat denaturation. Biochim Biophys Acta. 1969 Jan 21;174(1):71–85. doi: 10.1016/0005-2787(69)90230-5. [DOI] [PubMed] [Google Scholar]
- Van Holde K. E., Brahms J., Michelson A. M. Base interactions of nucleotide polymers in aqueous solution. J Mol Biol. 1965 Jul;12(3):726–739. doi: 10.1016/s0022-2836(65)80323-0. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Samejima T. Optical rotatory dispersion and circular dichroism of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1969;9:223–300. doi: 10.1016/s0079-6603(08)60770-9. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Janeway C. M., Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]


