Figure 1.
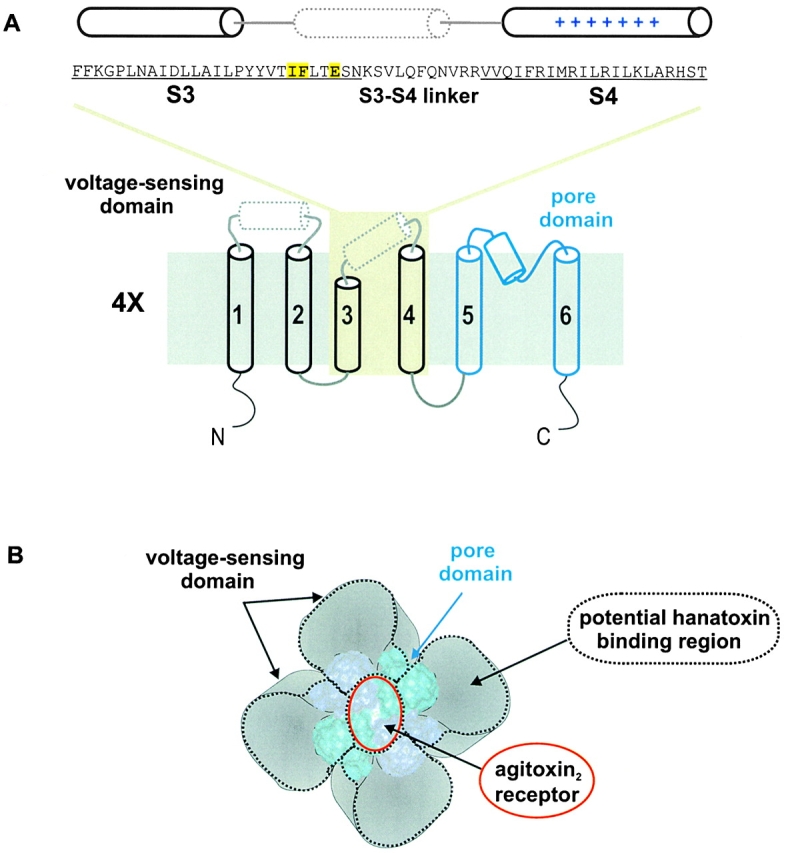
Topology and architecture of voltage-gated K+ channels. (A) Sequence of the drk1 K+ channel for the region starting at the NH2 terminus of S3 and ending at the COOH-terminal of S4 and the putative topology of a single α subunit. Yellow highlighting indicates the three positions (I273, F274, and E277) where point mutations have been found to alter the binding affinity of Hanatoxin (Swartz and MacKinnon 1997b). The putative demarcations of the S3 and S4 transmembrane segments, indicated by underlining, are based on Kyte-Doolittle hydrophobicity analysis. The cylinders above the sequence indicated the approximate positions of three α helices. The solid cylinders (for S3 and S4) are based on α-helical periodicity detected using alanine- and tryptophan-scanning mutagenesis (Hong and Miller 2000; Li-Smerin et al. 2000a). The dotted cylinder positioned near the COOH terminus of S3 and the S3–S4 linker was proposed based on helical periodicity detected with alanine-scanning mutagenesis (Li-Smerin et al. 2000a). The topology of a single K+ channel subunit shown (bottom) illustrates the first four transmembrane segments (S1–S4, in black) comprising the voltage-sensing domain and the last two transmembrane segments (S5 to S6, in blue) the pore domain. The dotted cylinder in the S1–S2 linker is based on helical periodicity detected with alanine-scanning mutagenesis (Li-Smerin et al. 2000a). The top is extracellular and the bottom is intracellular. (B) Architecture of voltage-gated K+ channels containing a central pore domain and four surrounding voltage-sensing domains drawn according to Li-Smerin et al. 2000b. The pore domain is represented by the crystal structure of KcsA (Doyle et al. 1998), a simple K+ channel homologous to the S5–S6 region of voltage-gated K+ channels. Adjacent pore domain subunits in the tetramer are illustrated using different shades of blue. The red ring over the ion conduction pore indicates the footprint of Agitoxin2 (∼20 × 30 Å), a pore-blocking toxin (MacKinnon et al. 1998). Each surrounding voltage-sensing domain is formed by S1–S4 transmembrane helices. The four areas surrounded by black dotted lines are regions that potentially interact with Hanatoxin, based on cohabitation of Hanatoxin and Agitoxin2. The overall dimensions of the channel are drawn using estimates of 80 × 80 Å from electron microscopy (Li et al. 1994).
