Figure 9.
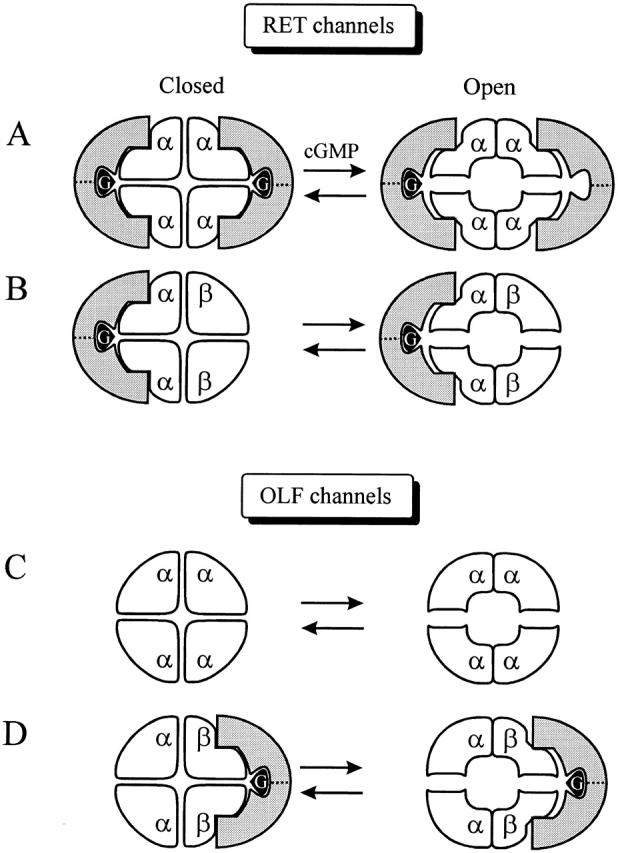
Schematic diagram of genistein inhibition. Homomeric and heteromeric RET and OLF channels and their interactions with PTKs, symbolized with the shaded crescent symbol. Genistein (G) is depicted bound to the PTK. In the closed state of the homomeric RETα channel (A), two PTKs, and thus two genistein molecules, are required to completely inhibit the channel. cGMP triggers channel opening, which functionally dimerizes RETα subunits. With the PTK spanning two functional dimers, only one PTK, and therefore one genistein, is sufficient to inhibit the channel, although two are still able to bind. In addition, the affinity of the closed channel for the PTK is higher than for the open channel, hence a tighter fit between the channel and the PTK symbol is depicted for the closed channel. For the RETα+β channel (B), only the RETα binds the PTK. Hence there is no change in the number of PTK required to blocked the closed and open channels. The homomeric OLFα channel (C) is unaffected by genistein because OLFα subunits do not contain PTK binding sites. The heteromeric OLFα+OLFβ (D) channel binds one PTK, via the binding sites on the OLFβ subunits.
