Figure 1.
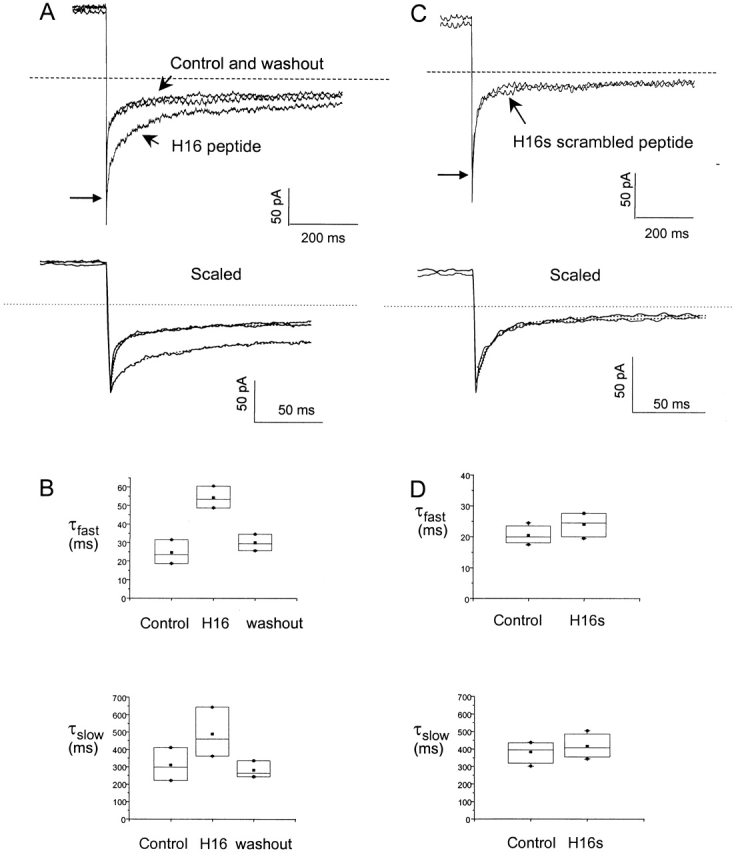
A soluble peptide corresponding to the first 16 amino acids (H16) reversibly restores slow deactivation to HERG channels lacking amino termini. (A) Tail currents from S620T Δ2-354 channels at −140 mV after a 1-s voltage step to 60 mV. (See methods regarding use of the S620T background.) Three current traces are shown, immediately after the patch was excised into the internal bath solution, after a 3-min incubation with 1 mM H16 peptide solution, and after a 5-min washout with peptide-free internal bath solution, respectively. The initial tail current amplitude, indicated by the long arrow, is unaffected by application of the peptide. The bottom panel, with expanded time scale, shows the initial tail current amplitude and includes dotted lines showing the fit with two exponential components. (B) Fast and slow mean time constants of deactivation extracted from exponential fits to the deactivating tail currents as shown in A. Values are τfast = 24.5 ± 6.5 ms, τslow = 309.8 ± 95.4 ms, A f (weight of the amplitude of the fast component represented as a percentage of total current) = 82.0 ± 8.2% for control; τfast = 54.2 ± 5.9 ms, τslow = 488.2 ± 143.5 ms A f = 73.4 ± 9.3% with the added peptide; and τfast = 29.9 ± 4.5 ms, τslow = 280.1 ± 48.3 ms, A f = 75.6 ± 6.9% upon washout of the peptide (n = 4). Using ANOVA (P < 0.01, at confidence interval = 0.05), there is a significant difference between the control and the experiment with peptide applied. (C) Tail currents before and after a 10-min incubation in the scrambled H16s peptide solution. (D) The mean time constants of both fast and slow components of the deactivation was not significantly changed by the scrambled peptide (ANOVA, P > 0.3 at confidence interval = 0.05). Values are τfast = 20.4 ± 2.9 ms, τslow = 381.9 ± 62.0 ms, A f = 89.7 ± 5.5% for control; τfast = 24.0 ± 4.2 ms, τslow = 414.7 ± 68.5 ms, A f = 81.6 ± 7.6% for the scrambled peptide (n = 3).
