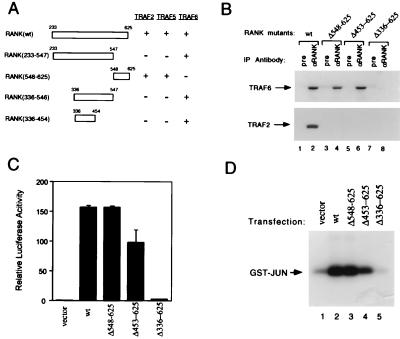
Figure 4.
RANK interactions with TRAF proteins and activation of NF-κB and JNK pathways. (A) Mapping of TRAF-binding domains of RANK. Expression vectors encoding full-length or deletion mutants of RANK intracellular domain fused to the GAL4 DNA-binding domain were cotransformed into HF7C yeast strain with vectors expressing the GAL4 activation domain fused with TRAF2, TRAF5, or TRAF6 as described (21). +, Growth after 1 week on the selection plates. (B) Coimmunoprecipitation of RANK and TRAF proteins. After 24 hr of transfection, 293 cell lysates were immunoprecipitated with pre-immune serum or Ab against RANK extracellular domain. Coprecipitating TRAF2 or TRAF6 were detected by immunoblot analysis with goat anti-TRAF2 or anti-TRAF6 Ab (Santa Cruz Biotechnology). (C) Activation of NF-κB in RANK transfected 293 cells. Luciferase activities were measured after 24 hr and normalized on the basis of β-galactosidase expression levels. The values (means ± SD) represent luciferase activities relative to vector transfection for representative experiments performed in duplicate. (D) Activation of JNK in RANK-transfected 293 cells. After 24-hr transfection, 293 cell lysates were immunoprecipitated with monoclonal anti-HA epitope Ab. Immunoprecipitates were assayed for kinase activity by using 2 μg GST-JUN (Stratagene) as substrate, and resolved by SDS/PAGE.