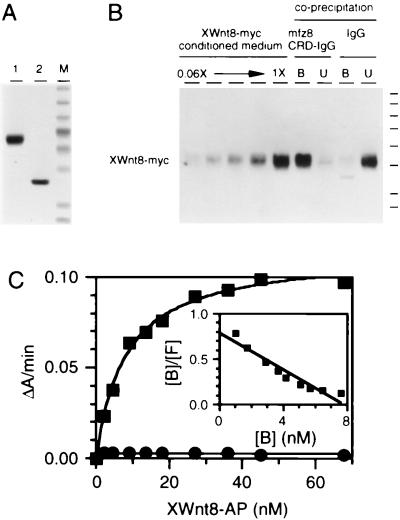
Figure 4.
Binding of XWnt8-myc and XWnt8-AP to mfz8CRD-IgG. (A) Coomassie-stained gel with mfz8CRD-IgG (lane 1) or the IgG fusion partner alone (consisting of the hinge and Fc regions) (lane 2) secreted from transfected 293 cells and purified by using protein A Sepharose. Molecular mass standards are 194, 120, 87, 64, 52, 39, 26, 21, and 15 kDa. (B) Binding efficiency of XWnt8-myc with IgG or mfz8CRD-IgG prebound to protein A Sepharose beads. Equal fractions of the bound (B), unbound (U), or starting material (1X) were analyzed by SDS/PAGE and anti-myc immunoblotting. A 2-fold dilution series of the starting material is shown at left. Greater than 90% of XWnt8-myc was bound to mfz8CRD-IgG, with little or no binding to IgG. Molecular mass standards are 194, 120, 87, 64, 52, 39, 26, and 21 kDa. (C) Solid phase binding of XWnt8-AP to IgG (circles) or mfz8CRD-IgG (squares). The rate of hydrolysis of p-nitrophenyl phosphate (measured as the rate of change in absorbance at 405 nm) is plotted against XWnt8-myc concentration. Inset shows a Scatchard plot of the binding data fit to a single straight line, which yields a calculated Kd of 8 nM. B, bound; F, free.