Abstract
During embryonic development chick and mouse spinal cords are activated by highly rhythmic episodes of spontaneous bursting activity at very early stages, while motoneurons are still migrating and beginning to extend their axons to the base of the limb. While such spontaneous activity has been shown to be important in refining neural projections once axons have reached their targets, early pathfinding events have been thought to be activity independent. However, in-ovo pharmacological manipulation of the transmitter systems that drive such early activity has shown that early motor axon pathfinding events are highly dependent on the normal pattern of bursting activity. A modest decrease in episode frequency resulted in dorsal-ventral pathfinding errors by lumbar motoneurons, and in the downregulation of several molecules required to successfully execute this guidance decision. In contrast, increasing the episode frequency was without effect on dorsal-ventral pathfinding. However, it prevented the subsequent motoneuron pool specific fasciculation of axons and their targeting to appropriate muscles, resulting in marked segmental pathfinding errors. These observations emphasize the need to better evaluate how such early spontaneous electrical activity may influence the molecular and transcription factor pathways that have been shown to regulate the differentiation of motor and interneuron phenotypes and the formation of spinal cord circuits. The intracellular signaling pathways by which episode frequency affects motor axon pathfinding must now be elucidated and it will be important to more precisely characterize the patterns with which specific subsets of motor and inter-neurons are activated normally and under conditions that alter spinal circuit formation.
Keywords: Spontaneous activity, Motoneuron pathfinding, Motor circuits, GABA, Glycine
1. Introduction
Many parts of the developing nervous system, including the retina and spinal cord, exhibit rhythmic bursts of spontaneous activity (O'Donovan, 1999; Torborg and Feller, 2005). This activity, which occurs at very early stages of development, consists of propagating waves that strongly activate many of the cells within the developing circuits. It differs from the activity that occurs spontaneously in late embryonic chick cord and which can be induced by drugs in peri-natal mouse cord. That more mature form of activity resembles adult locomotor patterns with alternating activation of flexor and extensor muscles and of right and left limbs during the bursting episodes (Cazalets et al., 1995; Whelan et al., 2000; Kiehn, 2006). In contrast, the early activity (E3-4 chick; E11.5-12.5 mouse) which is generated in isolated lumbar cords even in the absence of descending and afferent input, consists of propagating waves that cause near synchronous activation of most motoneurons on both sides of the cord. Additional differences are that in contrast to the late embryonic circuits, acetylcholine rather than glutamate provides the main excitatory drive, and GABA and glycine, the main inhibitory transmitters of the adult CNS, are both excitatory (Nishimaru et al., 1996; Hanson and Landmesser, 2003, 2004). As in many parts of the developing nervous system this is due to high levels of intracellular chloride in young neurons, resulting in Cl− efflux and thus depolarization when GABA and glycine receptors are activated (Kulik et al., 2000).
Is such early activity simply an epiphenomenon that is produced as developing circuits begin to assemble and become active or does it play an essential role in the development of these circuits? In this regard, are the unique attributes of the early activity, specifically its highly rhythmic nature, near-synchronous activation of many of the neurons in the circuit, and the long (2-4 minute) intervals between bursting episodes relevant? Many aspects of neuromuscular development are sensitive to activity levels including motoneuron survival, intramuscular nerve branching, and synaptogenesis (Moody and Bosma, 2005). However these activity-dependent events occur later in development after motor axons have begun to innervate their target muscles. In the visual system spontaneous retinal waves are required for the refinement of connections once axons have reached their targets within in the lateral geniculate nucleus, the superior colliculus/tectum, and the visual cortex (Torborg and Feller, 2005). However the general consensus in the field has been that early events such as axon growth to the target depends on a complex series of molecular signals but is independent of activity (Katz and Shatz, 1996; Sengpiel and Kind, 2002).
This review will highlight several recent studies that have demonstrated an unusual sensitivity of chick motor axon pathfinding on the precise pattern of spontaneous episodes of activity. Modest decreases in the frequency of bursting episodes were found to perturb the binary dorsal-ventral pathfinding decision at the base of the limb bud. In contrast modest increases in frequency prevented the subsequent motoneuron pool-specific growth of axons to their appropriate muscles (Hanson and Landmesser, 2004, 2006). Observations from other systems that demonstrate effects of activity on early developmental events will be considered and potential mechanisms through which activity may affect these events will be explored.
2. Mechanisms underlying the generation of spontaneous rhythmic bursting in early chick spinal cord
Although many studies have characterized aspects of spontaneous rhythmic bursting in rodent and chick cords (Nishimaru et al., 1996; Milner and Landmesser 1999; Branchereau et al., 2000; Hanson and Landmesser, 2003; Ren and Greer, 2003; Yvert et al., 2005) the most extensive delving into their mechanism of generation was carried out by O'Donovan and colleagues using a combination of electrophysiology and Ca2+ imaging (reviewed in O'Donovan et al., 1998). The E10 chick cord contains many recurrent excitatory connections between motoneurons and ventrally located interneurons. Individual motoneurons in the circuit spontaneously depolarize and when a sufficient number are activated, threshold for activation of the entire network is reached, resulting in rapid depolarization of the circuit and the generation of a wave of activity that propagates from the initiation site. The cells that initiate the wave at E10 are a group of Renshaw cell-like interneurons just dorsal to the lateral motor column and which are themselves excited by cholinergic inputs from the motoneurons. Intriguingly when the activation of this group of cells is pharmacologically blocked, other neurons take over to drive the activity episodes (Wenner and O'Donovan 2001). Thus the episodes of activity of early circuits are robust and difficult to perturb. As examples, when glutamate transmission, the main excitatory drive for spontaneous activity at E10, is blocked with NMDA and AMPA receptor antagonists, spontaneous activity ceases for several hours but then resumes, now being driven by GABA acting in an excitatory manner (Chub and O'Donovan, 1998). At E3-4, when ACh provides the main excitatory drive, nicotinic antagonists block activity for only 20-60 minutes, and when activity returns it is driven by GABA acting via GABAA receptors. Thus bicuculline, a GABAA antagonist, blocks this resumed activity while having only a modest effect on a control cord prior to any drug exposure (Milner and Landmesser, 1999).
In both E10 and E3-4 chick cord and in the E11.5-12.5 mouse cord, following an episode of activity, a period of circuit depression occurs, during which it is impossible to elicit a propagating episode, even by exogenous electrical stimulation. However, as the depression declines, one can elicit prematurely a full episode of activity by a single stimulus to the cord (Hanson and Landmesser, 2003). The interval between episodes is therefore normally determined by the gradually increasing excitation of the circuit and the decline in circuit depression. While many as yet uncharacterized mechanisms may contribute to circuit depression, the large efflux of Cl− during an activity episode, with the resultant shift in the Cl− equilibrium potential to more negative values, has been proposed and shown by modeling to be able to account for many experimentally observed characteristics of the pattern of spontaneous activity (Marchetti et al., 2005). Recently, measurements of intracellular Cl− via a fluorescent indicator have provided support for this model (Chub et al., 2006). Although the circuit that generates spontaneous activity at E3-4 (chick) and E11.5-12.5 (mouse) differs from the more mature E10 chick or E 18.5 mouse circuit, notably in that it is the motoneurons themselves via the release of acetylcholine that provide the main excitatory drive (Milner and Landmesser, 1999, Hanson and Landmesser 2003), the basic organization of the circuit that allows it to produce highly rhythmic strong depolarizations appears similar at both developmental stages. As shown below, maintaining the normal frequency of the activity episodes is required for motor axons to successfully execute both dorsal-ventral and pool-specific pathfinding decisions.
3. Limb innervating motoneurons make two distinct major pathfinding decisions: a binary dorsal-ventral choice and the subsequent selective fasciculation and growth to the appropriate muscle of all axons belonging to each motoneuron pool
The first choice, to project dorsally or ventrally, is made very early as motor axons growing out different spinal nerves begin to converge in the plexus at the base of the limb bud. Although initially intermingled, axons from medially (LMCm) and laterally (LMCl) situated motoneurons segregate from one another even before the spinal nerves converge (Lance-Jones and Landmesser, 1981a) and as the spinal nerves defasciculate at the plexus, they project either dorsally or ventrally (Fig 1A shows the axons from two dorsal LMCl pools, the femorotibialis (red) and the sartorius (green) segregating from the blue, ventrally projecting LMCm adductor axons). Ephrin/EphA4, GDNF/Ret, and semaphorin/neuropilin signaling pathways all contribute to this guidance decision (Eberhart et al., 2002; Kania and Jessell, 2003; Kramer et al., 2006; Huber et al., 2005). In addition, the defasciculation of the axons at the plexus mediated by polysialic acid on NCAM, has been shown to play a permissive role in allowing the axons to respond to the D-V guidance cues (Tang et al., 1992,1994).
Figure 1. Major pathfinding decisions of lumbar spinal motor axons.
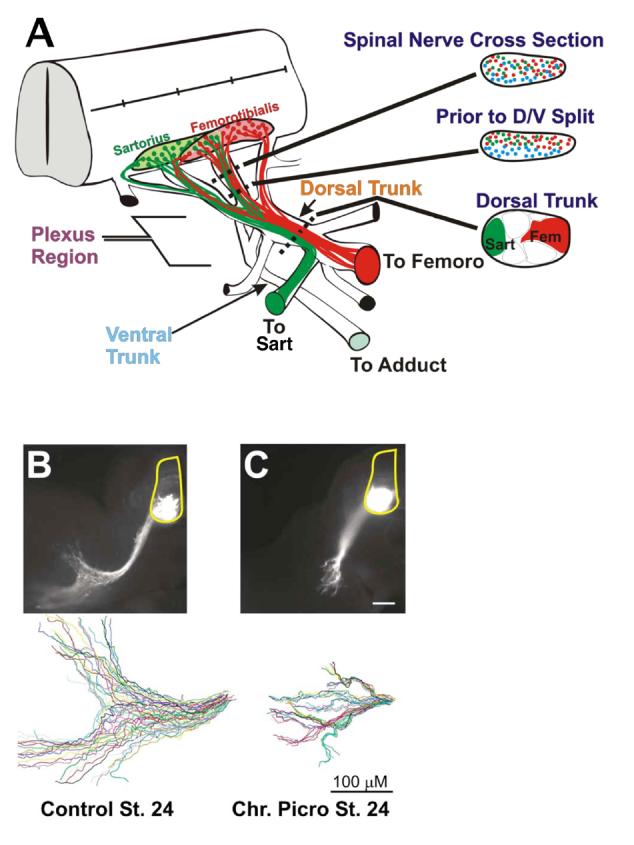
A. Drawing that illustrates the sorting of LMCl dorsally projecting femorotibialis (red) and sartorius (green) motor axons from LMCm ventrally projecting adductor (blue) motoneurons in the crural pleuxus. After this dorso-ventral (D-V) sorting, the two dorsal pools segregate into discrete fascicles. The medially located adductor pool is not shown in cord for clarity. Femoro, femorotibialis; sart, sartorius; adduct, adductor. B,C. patterns of defasiculation of motor axons in the plexus in control and picrotoxin treated embryos respectively. Top-thick vibratome slices transverse to cord with motor axons visualized by eGFP under the control of a motoneuron specific promotor. Bottom- single color coded axon trajectories traced with neurolucida software from slices above.
Since motoneuron pools extend over 2-4 segments of cord, the next pathfinding decision requires that all the axons that belong to an individual motoneuron pool segregate from their neighbors in the different spinal nerves and selectively fasciculate, as shown by the green sartorius and red femorotibialis axons in Fig 1A. These fascicles subsequently diverge from nerve trunks in response to target-derived guidance cues to reach their appropriate destinations in the limb bud mesenchyme. Hox genes and their cofactors have recently been shown to specify the identity of some motoneuron pools, including their peripheral connections (Dasen et al., 2005), but the downstream guidance molecules that enable a motoneuron pool to reach its appropriate muscle are largely unknown. Previous embryonic surgical manipulations provided support for the idea that axons were responding to diffusible chemoattractants from target regions (Lance-Jones and Landmesser, 1981b) and recently FGF (fibroblast growth factor) has been identified as a chemoattractant that attracts medial motor column axons to their axial muscles (Shirasaki et al., 2006).
Since limb innervating motoneurons are activated by rhythmic bursts of spontaneous activity while in the process of making both the D-V and the motoneuron pool specific guidance decisions (Milner and Landmesser, 1999; Hanson and Landmesser, 2004), we sought to determine if this activity was required for accurate pathfinding. To explore this possibility, chick embryos were treated for 2 days (from Stage 20-25 of Hamburger and Hamilton, E3-4) with twice daily injections of drugs that had been found to alter the frequency of bursting episodes in Stage 23-25 isolated cord preparations; picrotoxin, a GABAA receptor antagonist, caused the intervals between episodes to increase from 2 to 4 minutes (Hanson and Landmesser, 2004), while sarcosine, an antagonist of the GlyT1 glycine transporter (Eulenberg et al., 2005), caused the interepisode interval to decrease to 1 minute (Hanson and Landmesser, 2006). The sarcosine induced increase in the frequency of activity was proposed to occur through the enhanced action of endogenously released glycine, which is excitatory at these developmental stages in both chicks and rodents (Wu et al., 1992; Nishimaru et al., 1996; Milner and Landmesser, 1999; Kulik et al., 2000). Indeed, the alterations in the frequency of spontaneous activity episodes produced by sarcosine and the resultant disruption of pool-specific pathfinding, described below, were prevented by simultaneous treatment with the glycine receptor antagonist strychnine (Hanson and Landmesser, 2006). Although glycine is also known to modulate NMDA receptor activation (Konradsson et al., 2006) neither NMDA nor AMPA receptor antagonists affect early spontaneous activity in the chick (Milner and Landmesser, 1999). Thus it is unlikely that the effects of sarcosine could be due to glycine acting on NMDA receptors. Finally, even though limb muscles have not yet formed during the period of in-ovo drug treatments, it was possible to confirm that picrotoxin and sarcosine affected spontaneous activity in the intact developing embryos similarly to their effects on isolated cords, because they altered the frequency of axial movements, as axial muscles are innervated at this stage.
4. Decreasing the frequency of spontaneous bursting episodes results in dorsalventral pathfinding errors
Following chronic treatment with picrotoxin, which reduced the frequency of bursting episodes by half, motor axons converged in the crural plexus and despite a less robust defasciculation during axon sorting in the plexus (Figure 1B,C), formed an anatomically normal pattern of muscle nerves that by E7 had innervated both dorsal and ventral muscles (Hanson and Landmesser, 2004). However retrograde labeling of the dorsal femorotibialis and sartorius and the ventral adductor muscles, revealed that approximately 10-15% of motoneuron somas in each pool were in inappropriate medial-lateral locations for the muscle to which they projected. As an example some neurons projecting to the sartorius were located medially in the position of the adductor pool. To distinguish if such misplaced motoneuron somas were adductor motoneurons that had made D-V pathfinding errors or were sartorius motoneuron somas that had failed to migrate properly, their expression of LIM homeobox genes, which have been shown to define the LMCl and LMCm columnar identity of motoneurons (Shirasaki and Pfaff, 2002), was determined. All misplaced motoneuron somas were found to result from D-V pathfinding errors. However no errors in A-P pathfinding were detected and the three muscles studied in detail were innervated by segmentally correct motoneurons.
The retinal wave dependent refinement of connections in the visual system is thought to involve competitive Hebbian or spike timing dependent interactions of retinal afferents and requires activation of the postsynaptic cell (Mu and Poo, 2006). However, motor axons are just growing into the limb bud at the time of the activity alterations and limb muscles have not yet formed. Another way in which activity could influence pathfinding is by altering the axonal expression of receptors for guidance cues or the ability of growth cones to respond to such cues. Two molecules, which are required for accurate D-V pathfinding, the ephrin receptor EphA4 (Eberhart et al., 2002; Kania and Jessell, 2003) and polysialic acid on NCAM (Tang et al., 1992) were in fact strongly downregulated on the motor axons by sarcosine treatment (Hanson and Landmesser, 2004). To prove that the downregulation of either of these molecules was responsible for the D-V pathfinding errors, would require a demonstration that maintaining their expression levels even with the alteration in activity would rescue the observed pathfinding errors. While this has not yet been achieved, the similarity in both the amount and type of D-V pathfinding errors and the pattern of axon fasciculation in the plexus with both sarcosine treatment and with the enzymatic removal of polysialic acid (Tang et al., 1992, 1994), suggests that an alteration in polysialic acid expression is likely to underlie the D-V guidance errors.
5. Increasing the frequency of spontaneous bursting episodes strongly disrupts A-P or pool-specific pathfinding
Chronic treatment with the GLYT1 transporter sarcosine, which increased the frequency of bursting episodes during the time that axons were making both D-V and pool-specific pathfinding decisions, had no effect on the accuracy of D-V pathfinding. Sarcosine treatment also had no effect on the expression of polysialic acid or EphA4. However it strongly disrupted the fasciculation of axons belonging to the same pool that originated in different spinal cord segments. (Hanson and Landmesser, 2006). As shown in Figure 2, lumbosacral (LS) spinal nerves1-3 still converged to from a morphological plexus. However retrograde labeling with lipophilic dyes or fluorescent dextrans, revealed that the intermingling of axons from different motoneuron pools, as seen in the control sartorius (green) and femorotibialis (red) axons in LS2, failed to occur. Instead, most of the dorsal axons in LS2 innervated the femorotiibialis, with none projecting to the sartorius. Similarly, most of the dorsal axons in LS1 projected only to the sartorius. This altered innervation was apparent in limb bud whole mounts of labeled axons (Fig. 2a), as well as from quantification of the segmental localization of motoneuron somas (Fig. 2b). Note for example that the control femorotibialis pools is located in LS1,2, and 3 whereas in the sarcosine treated embryo neither LS1 nor LS3 contributeed to the femorotibialis innervation. Similarly the LS2 contribution to the sartorius failed to occur. These alterations in segmental innervation patterns were also detected from EMG recordings following spinal nerve stimulation (Fig. 2c). These results suggested that each muscle was now innervated by a mixture of its own motoneurons and by foreign motoneurons belonging to the other pool.
Figure 2. Altered A-P/motoneuron-pool specific pathfinding following sarcosine treatment.
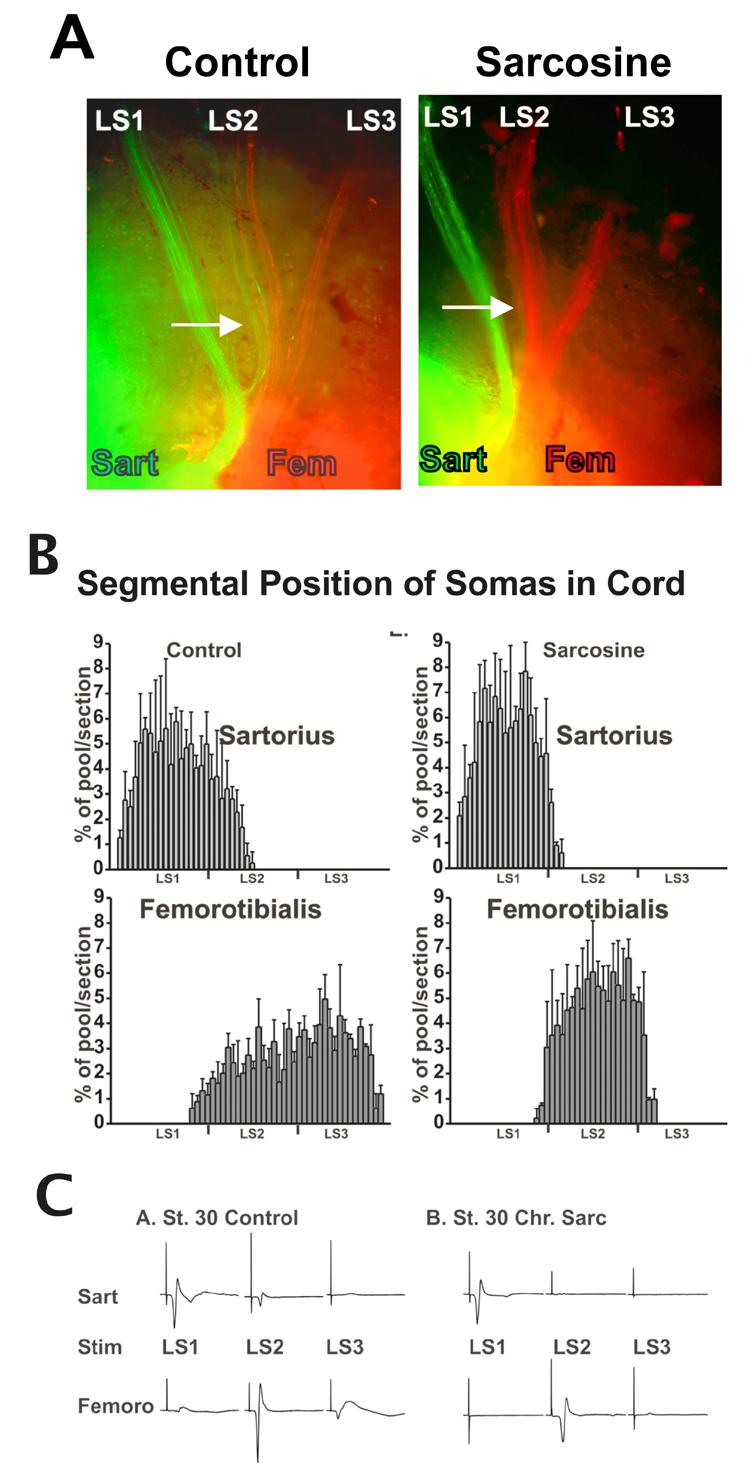
A. Whole mounts of axons retrogradely labeled with fluorescent dextrans injected into the sartorius (green) and the femorotibialis (red) muscles in control and sarcosine treated embryos. No dually labeled axons were ever observed in the control or sarcosine treated cases. The slight yellow glow in the sarcosine treared example is due to glare from the injection site. B. Histograms showing the A-P localization of motoneuron somas for the sartorius and femorotibialis pools in control and sarcosine treated embryos, expressed as per cent of pool/section. C. Compound action potentials from EMG recordings from the sartorius and femorotibialis muscles following electrical stimulation of lumbosacral (LS) spinal nerves 1, 2, or 3. A-P, anterior-posterior.
Since the sartorius is a flexor and the femorotibilalis an extensor, these pools are normally activated out of phase (Landmesser and O'Donovan, 1984a). Because it was previously shown that the activation patterns of motoneuron pools were not altered when they innervated foreign muscles (Landmesser and O'Donovan, 1981b), these activation patterns can be used to determine the identity of the innervating motoneurons. In sarcosine treated embryos these activation patterns revealed that each muscle was now being innervated by a mixture of sartorius and femorotibialis motoneurons, which had maintained their original bursting patterns (Hanson and Landmesser, 2006). Thus following sarcosine treatment, pool-specific pathfinding errors were made but the identity of motoneuron pools, as judged by their pool-specific bursting patterns, was not altered.
The A-P pathfinding errors described above occurred at the time of plexus formation and pool-specific axon sorting, which is between Stage 23-25. To determine if the pathfinding errors were permanent or if they could be corrected upon resumption of activity, sarcosine treatment was halted at Stage 24, with activity returning to control levels by Stage 25. At Stage 30 (E7) when muscles are initially innervated, retrograde labeling of the axons projecting to the sartorius and femorotibialis muscles revealed that the pathfining errors had largely been corrected, based on axon tracing, spinal nerve stimulation, and the position of motoneuron pools within the spinal cord (Hanson and Landmesser, 2006). These corrections occurred distal to the plexus by axons forming novel nerves to reach their appropriate muscles. This observation provides additional evidence that the identity of the motoneuron pools had not been altered. It also demonstrates that motoneuron growth cones are sensitive to the patterns of activity imposed on them throughout their growth to their muscle targets and that they are likely responding to diffusible signals from the target region.
The molecular mechanisms underlying the pool-specific pathfinding errors are unknown as is the basis for normal pool specific pathfinding. However since this type of pathfinding error was widespread, even though only two pools were studied in detail, it appears unlikely that the many molecules required for different motoneuron pools to correctly pathfind would all have their expression altered. We therefore favor the hypothesis that a common intracellular signaling step that is used by all motoneuron growth cones to respond to different target derived chemoattactants is being affected. In cultured retinal ganglion cells a 2 Hz 5 second train of electrical stimuli increases Ca2+ levels in the growth cone and causes it to be attracted rather than repulsed by myelin associated glycoprotein. Ten stimuli at 2 Hz are also sufficient to lower the threshold for detecting the chemoatractant netrin (Ming et al., 2001). Thus the altered frequency of motoneuron activation may have simply prevented growth cones from responding to target derived guidance cues, until the normal patterns of activity were restored.
6. Discussion and Future Directions
A series of recent studies (McLaughlin at al., 2003; Cang et al., 2005; Chandrasekaran et al., 2005; Huberman et al., 2006) have confirmed and extended earlier work from the Shatz laboratory (Penn at al., 1998; Stellwagen and Shatz, 2002) in clearly demonstrating that spontaneous retinal waves are required for the refinement of visual projections in multiple target structures; the lateral geniculate nucleus, the superior colliculus, and the cortex. The work described here indicates that in developing spinal circuits, similar rhythmic wavelike activity is required at even earlier developmental stages and is required for motor axons to successfully execute both dorsal-ventral and pool-specific pathfinding. Whether activity is also required in the visual system for guidance of axons to their targets has not yet been investigated.
In both systems it appears that a salient feature of the activity is the strong, spaced depolarizations rather than overall levels of activity (Torborg et al., 2005; Hanson and Landmesser, 2004). Such patterns of activity are known to be highly effective in modulating gene expression (Dolmetch et al., 1997; Wu et al., 2001) and the frequency, amplitude, and the path of entry of Ca2+ during the Ca2+ transients that accompany such depolarizations can determine which genes or signaling pathways are activated (West et al., 2001; see Moody and Bosma, 2005 for review). Indeed, the frequency of Ca2+ transients has been shown to be critical in regulating the expression of GAD 67 and thus the GABAergic phenotype in dissociated spinal neurons (Watt et al., 2000) and the expression of various adhesion molecules in cultured sensory neurons (Itoh et al.,1997). Such signals may be important not only in the soma but also in the growth cone where they could modulate gene translation, the stabilization or removal of specific receptors from the cell surface, or the cytoskeleton.
While these observations from reduced systems provide important insights into potential signaling mechanisms, an important distinction is that in-vivo neurons are embedded within complex circuits. Thus in the case of early motoneurons, when a spontaneous bursting episode occurs, they will not only experience a large rise in Ca2+ but they will also be exposed simultaneously to a number of neurotransmitters (ACh, GABA and glycine) which can potentially activate a number of receptor subtypes that are expressed on motoneurons at that time (Figure 3). During the asynchronous firing of individual motoneurons that occurs between bursting episodes, and which increased when picrotoxin reduced the frequency of bursts so that overall times a motoneuron was activated per unit time was not altered (Hanson and Landmesser, 2004), motoneurons would exhibit weaker Ca2+ transients and would not be strongly stimulated by transmitters. In addition, neurotrophins such as BDNF are likely to be released during episodes. Thus an important question that needs to be resolved is whether it is the altered frequency of the spontaneous episodes that is affecting the two major pathfinding decisions carried out by motor axons. Alternatively, could the blockade of GABAA signaling by picrotoxin or the enhanced signaling via glycine receptors following sarcosine treatment be the critical feature? GABA and glycine, sometimes released in a paracrine fashion, can influence early events in neural development including proliferation and differentiation of neuronal precurors as well as cell migration (Nguyen et al., 2001; Moody and Bosma, 2005).
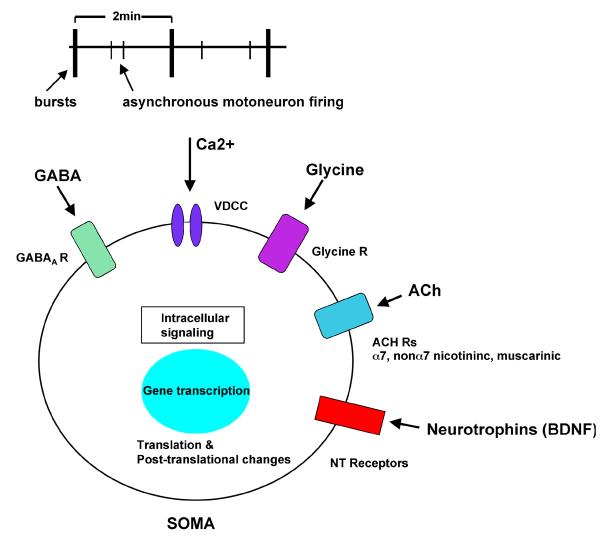
Figure 3. Possible signaling pathways that may mediate the effects of altered frequencies of spontaneous activity on motor axon pathfinding.
During bursts Ca2+ may enter through voltage dependent Ca2+ channels (VDCC), with L, P/Q, N, and T type channels all present. The transmitters GABA, glycine, and acetylcholine (ACh) will all be released and can act via their receptors which are present on motoneurons at early stages of development. α7 and non-α7 nicotinic receptors as well as muscarinic receptors are present. Motoneurons also express neurotrophin (NT) receptors such as TrkB which can be activated by activity dependent BDNF (brain derived neurotophic factor) release. During asynchronous firing of motoneurons between bursts Ca2+ influx would occur but motoneurons would not be as strongly activated by the transmitters and neurotrophins.
One way to distinguish between these alternatives is to activate the cord with the control pattern of activity while simultaneously blocking GABAA signaling with picrotoxin. If the D-V pathfinding errors were prevented this would implicate the frequency of activity episodes rather than altered GABAA transmission/signaling. Such in-ovo activation of circuits has become feasible with the development of light activated channels that can be genetically incorporated into specific subtypes of neurons. In-ovo electroporation of channelrhodopsin 2, a cation channel that can be activated with a specific wavelength of light, into Stage 18 chick neural tube, provided a means to precisely time the activation of propagating episodes of activity within intact embryos by applying flashes of light through a window in the shell (Li et al., 2005). Expresssing such channels, including those that lead to hyperpolarization, under the control of neuron subtype specific promoters will enable the activity of specific subsets of cells to be better controlled. However even with such advances it will remain difficult to tease apart the important variables in complex intact circuits. Another area of ignorance that needs to be investigated is how early and with what pattern are different subtypes of neurons activated by rhythmic depolarizations in relation to other important developmental events; proliferation, migration, axon extension, or expression of specific molecules. High resolution, multiphoton Ca2+ imaging provides a means to achieve this at a single cell level either in intact cords preparations or in cord slices (O'Donovan et al., 2005). Such approaches should enable a much better understanding of how the spontaneous rhythmic activity in developing neural circuits interacts with molecular signaling cascades to contribute to the formation of complex functional circuits.
Acknowledgements
supported by NIH grants NS19640 and NS23687
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Branchereau P, Morin D, Bonnot A, Ballion B, Chapron J, Viala D. Development of lumbar rhythmic networks: from embryonic to neonate locomotor-like patterns in the mouse. Brain Res. Bull. 2000;53:711–718. doi: 10.1016/s0361-9230(00)00403-2. [DOI] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J. Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J. Neurosci. 2005;25:6929–6938. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chub N, Mentis GZ, O'Donovan MJ. Chloride-sensitive MEQ fluorescence in chick embryo motoneurons following manipulations of chloride and during spontaneous network activity. J. Neurophysiol. 2006;95:323–330. doi: 10.1152/jn.00162.2005. [DOI] [PubMed] [Google Scholar]
- Chub N, O'Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J. Neurosci. 1998;85:294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Eberhart J, Swartz ME, Koblar SA, Pasquale EB, Krull CE. EphA4 constitutes a population-specific guidance cue for motor neurons. Dev. Biol. 2002;247:89–101. doi: 10.1006/dbio.2002.0695. [DOI] [PubMed] [Google Scholar]
- Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem. Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic spinal cord. J. Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;46:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Increasing the frequency of spontaneous rhythmic activity disrupts pool-specific axon fasciculation and pathfinding of embryonic spinal motoneurons. J. Neurosci. 2006;26:12769–12780. doi: 10.1523/JNEUROSCI.4170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, Johnson D, Jessell TM, Ginty DD, Kolodkin AL. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron. 2005;48:949–964. doi: 10.1016/j.neuron.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Speer CM, Chapman B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields. Neuron. 2006;52:247–252. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Ozaki M, Stevens B, Fields RD. Activity dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J. Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A: EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Ann Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Konradsson A, Marcus MM, Hertel P, Svensson TH, Jardenmark KE. Inhibition of the glycine transported GlyT-1 potentiates the effect of risperidone but not clozapine on glutamatergic transmission in the rat medial prefrontal cortex. Synapse. 2006;60:102–108. doi: 10.1002/syn.20286. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Knott L, Su F, Dessaud E, Krull CE, Helmbacher F, Klein R. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron. 2006;50:35–47. doi: 10.1016/j.neuron.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nishimaru H, Ballanyi K. Role of bicarbonate and chloride in GABA- and glycine- induced depolarization and [Ca2+]I rise in fetal rat motoneurons in situ. J. Neurosci. 2000;20:7905–7913. doi: 10.1523/JNEUROSCI.20-21-07905.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance-Jones C, Landmesser LT. Pathway selection by chick lumbosacral motoneurons during normal development. Proc. R. Soc. Lond. B. 1981a;214:1–18. doi: 10.1098/rspb.1981.0079. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, Landmesser LT. Pathway selection by embryonic chick lumbosacral motoneurons in an experimentally altered environment. Proc. R. Soc. Lond. B. 1981b;214:19–52. doi: 10.1098/rspb.1981.0080. [DOI] [PubMed] [Google Scholar]
- Landmesser LT, O'Donovan MJ. Activation patterns of embryonic chick hind limb muscles recorded in-ovo and in an isolated spinal cord preparation. J. Physiol. (Lond) 1984a;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser LT, O'Donovan MJ. The activation patterns of embryonic chick motoneurons projecting to inappropriate muscles. J. Physiol. (Lond) 1984b;347:205–224. doi: 10.1113/jphysiol.1984.sp015062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast non-invasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Tabak J, Chub N, O'Donovan MJ. Modeling spontaneous activity in the developing spinal cord using activity-dependent variations in intracellular chloride. J. Neurosci. 2005;25:3601–3612. doi: 10.1523/JNEUROSCI.4290-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. J. Neurosci. 1999;19:3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Henley J, Tessier-Lavigne M, Song HJ, Poo M-m. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;4:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol. Rev. 2004;85:883–941. doi: 10.1152/physrev.00017.2004. [DOI] [PubMed] [Google Scholar]
- Mu W, Poo M-m. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron. 2006;50:115–125. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Iizuka S, Ozaki S, Kudo N. Spontaneous motoneuronal activity mediated by glycine and GABA in the spinal cord of rat fetuses in vitro. J. Physiol. (Lond) 1996;497:132–143. doi: 10.1113/jphysiol.1996.sp021755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Rigo J-M, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr. Opin. Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Bonnot A, Wenner P, Mentis GZ. Calcium imaging of network function in the developing spinal cord. Cell Calcium. 2005;37:443–450. doi: 10.1016/j.ceca.2005.01.012. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Chub N, Wenner P. Mechanisms of spontaneous activity in developing spinal networks. J. Neurobiol. 1998;37:131–145. doi: 10.1002/(sici)1097-4695(199810)37:1<131::aid-neu10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Ontogeny of rhythmic motor patterns in the embryonic rat spinal cord. J. Neurophysiol. 2003;89:1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Kind PC. The role of activity in development of the visual system. Curr. Biol. 2002;12:R18–R26. doi: 10.1016/s0960-9822(02)01318-0. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Lewcock JW, Lettieri K, Pfaff SL. FGF as a target derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron. 2006;50:841–853. doi: 10.1016/j.neuron.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Ann. Rev. Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogenicuate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Tang J, Landmesser L, Rutishahuser U. Polysialic acid influences specific pathfinding by avian motoneurons. Neuron. 1992;8:1031–1044. doi: 10.1016/0896-6273(92)90125-w. [DOI] [PubMed] [Google Scholar]
- Tang J, Rutishauser U, Landmesser L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron. 1994;13:405–414. doi: 10.1016/0896-6273(94)90356-5. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat. Neurosci. 2005;8:72–78. doi: 10.1038/nn1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Progress in Neurobiology. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Watt SD, Gu X, Smith RD, Spitzer NC. Specific frequencies of spontaneous Ca2+ transients upregulate GAD 67 transcripts in embryonic spinal neurons. Mol. Cell. Neurosci. 2000;16:376–387. doi: 10.1006/mcne.2000.0871. [DOI] [PubMed] [Google Scholar]
- Wenner P, O'Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J. Neurophysiol. 2001;86:1481–1498. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MG, Dolmetsch RE, Kornhauser JM, Shawitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J. Neurophysiol. 2000;84:2821–2833. doi: 10.1152/jn.2000.84.6.2821. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat. Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Wu W, Ziskind-Conhaim L, Sweet MA. Early development of glycine- and GABA- mediated synapses in rat spinal cord. J. Neurosci. 1992;12:3935–3945. doi: 10.1523/JNEUROSCI.12-10-03935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]