Figure 1.
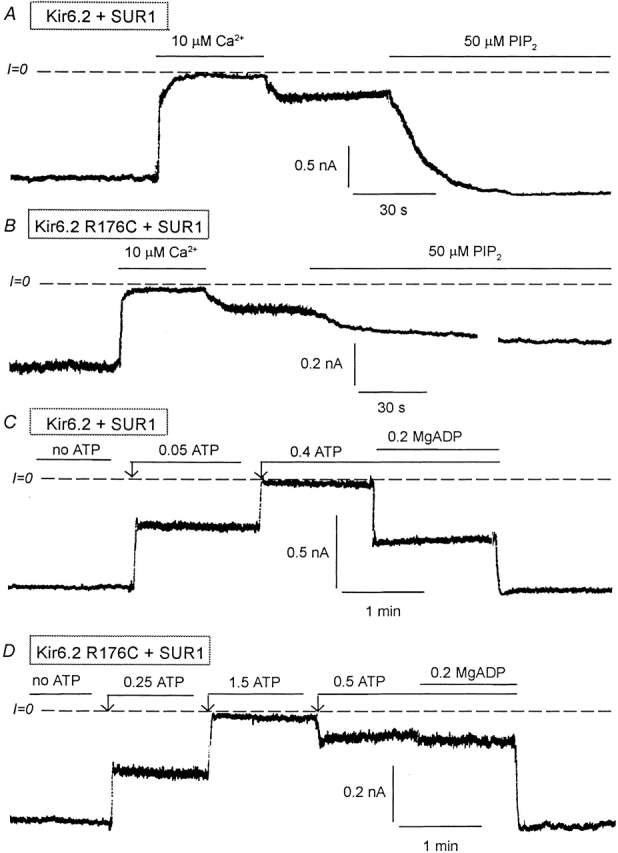
Effects of PIP2 and MgADP on Kir6.2 wild-type and R176C mutant (linked to GFP) coexpressed with SUR1. (A) Representative inward currents from excised inside-out patches with wild-type Kir6.2+SUR1. Brief application of 10 μM Ca2+ (for ∼1 min) to the cytoplasmic surface of an excised inside-out patch rapidly inhibited channel activity, which recovered only minimally after removal of Ca2+. PIP2 application led to full recovery of channel activity after brief Ca2+-induced rundown. Dashed lines indicate zero current level. (B) A similar experiment performed with the R176C mutant + SUR1 show a similar inhibitory effect due to Ca2+, but the response to PIP2 was very slow and, in this case, reached 42% of control after 8 min. The trace was interrupted for 3 min during the application of PIP2. (C) Wild-type Kir6.2+SUR1 inward currents were suppressed by increasing ATP concentrations. In the presence of 400 μM ATP, addition of 200 μM MgADP caused a robust stimulation of channel activity, indicating functional coupling between Kir6.2 and SUR1. (D) A similar inside-out patch experiment performed with the R176C mutant + SUR1 shows that in the presence of 500 μM ATP, addition of 200 μM MgADP had little or no effect indicating functional uncoupling of Kir6.2 and SUR1. In this case we first applied 1.5 mM ATP to show complete inhibition by ATP and we reduced the ATP level to 0.5 mM so that the effects of MgADP could be compared with that obtained with the wild-type channel.
