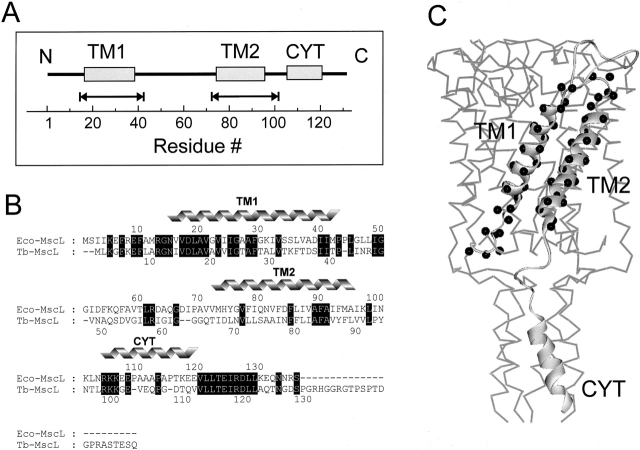
Figure 1.
(A) Linear representation of the membrane topology of Eco-MscL. The helical portions of the Eco-MscL monomer (the transmembrane domains TM1 and TM2 and the cytoplasmic helix CYT) are represented by rectangles. The scale corresponds to the amino acid residue numbering. The double-headed arrows designate the amino acid residues replaced by reactive cysteines used for spin labeling. (B) Sequence alignment of Eco-MscL and Tb-MscL. Identical residues are highlighted (black). The residues corresponding to two transmembrane helical domains (TM1 and TM2) and the cytoplasmic helix (CYT) are marked. (C) Single MscL subunit showing amino acid residues subjected to cysteine scanning mutagenesis (black spheres). The single MscL monomer is represented as a part of the channel pentamer according to the 3-D crystal structure of the channel.