Figure 6.
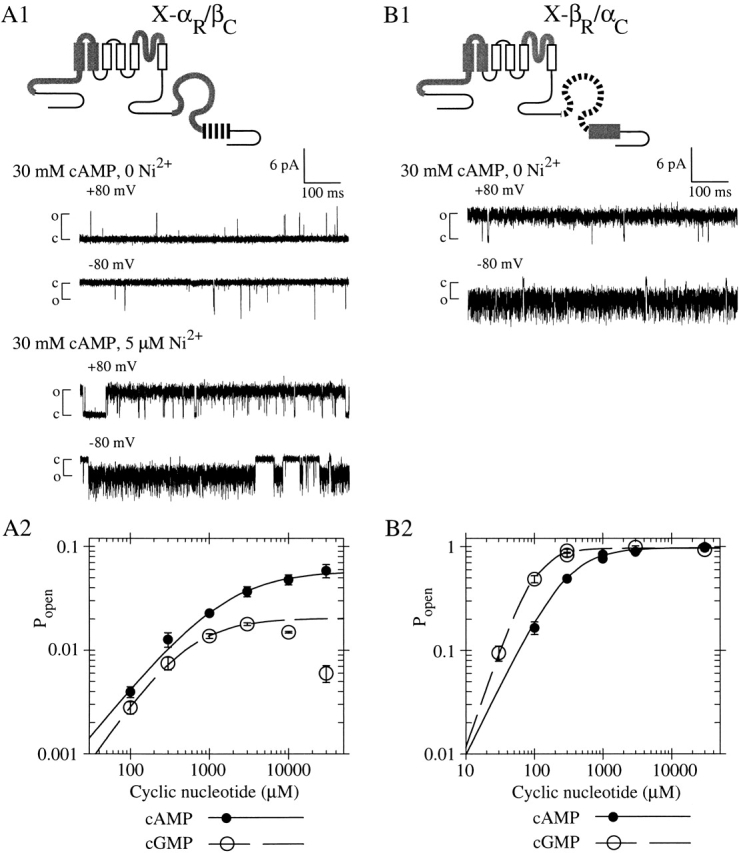
Chimera X-αR/βC is poorly activated by cyclic nucleotide, whereas the complementary chimera X-βR/αC is efficiently activated by cyclic nucleotide. (A1) X-αR/βC contains the roll subdomain from fCNG2 and the C-helix from rCNG5. Single-channel recordings were made at indicated potentials with a steady-state concentration of 30 mM cAMP, with or without 5 μM Ni2+ present. (top traces) Pmax is low in absence of Ni2+ (Popen = 0.004 at +80 mV and 0.012 at −80 mV). (bottom traces) Channel opening is potentiated by Ni2+, showing only one channel is present (Popen = 0.874 at +80 mV and 0.878 at −80 mV). (A2) Mean dose–response data for X-αR/βC activation by cAMP (closed circles) and cGMP (open circles) at −100 mV, compiled from four patches expressing macroscopic currents (materials and methods); graph is normalized using Pmax,cAMP = 0.056, the mean from single-channel measurements. Lines show Hill equation fits with parameters (±SEM) as follows: for cAMP (solid), K1/2 = 1,570 ± 370 μM, h = 0.932 ± 0.065; for cGMP (dashed, excluding data >3 mM), K1/2 = 511 ± 37 μM, h = 1.120 ± 0.044; Imax,cAMP/Imax,cGMP = 2.85 ± 0.30. (B1) X-βR/αC contains the roll subdomain from rCNG5 and the C-helix from fCNG2. Single-channel recording excerpt (no Ni2+ present) shows high Pmax in 30 mM cAMP (Popen = 0.9878 at +80 mV and 0.9896 at −80 mV). (B2) Dose–response data for X-βR/αC activation by cAMP (solid circles) and cGMP (open circles) at −100 mV, from a patch with maximal current ∼400 pA; graph is normalized using Pmax,cAMP = 0.954, the mean from single-channel measurements. Lines show Hill equation fits with parameters (±SE) as follows: for cAMP (solid), K1/2 = 288 ± 20 μM, h = 1.37 ± 0.12; for cGMP (dashed), K1/2 = 95.8 ± 9.2 μM, h = 1.95 ± 0.22; Imax,cAMP/Imax,cGMP = 1.0078 ± 0.030.
