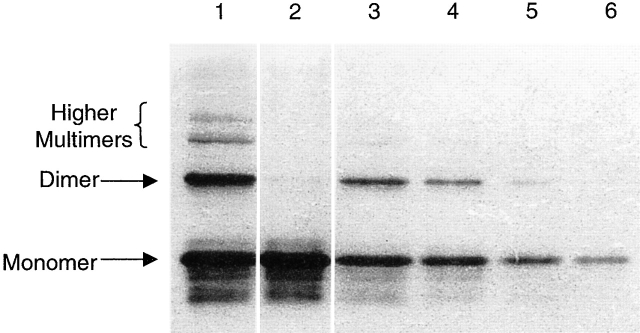
Figure 2.
Native 15% PAGE showing the disaggregation of preformed aggregates by DMSO. Lane 1 shows a heavily loaded (18 μg) sample of T domain with a COOH-terminal H6 tag. This sample was chosen for its unusually large dimer and multimer populations. (The band labeled “dimer” was so identified from its running at the same position as an unreduced T domain cysteine mutant.) Contrast this with lane 2, which shows the same quantity of protein after incubation in 40% DMSO for 2 h at 37°C. Notice the near absence of dimers and multimers. Lanes 3–6 are dilutions of lane 1 made to quantify the effects of the DMSO protocol. The dilutions are 6-, 12-, 30-, and 60-fold, respectively. Thus, it appears that >97% of the preformed dimers have been broken up. Samples of lanes 1 and 2 were also tested on bilayers and showed no noticeable difference in their ability to form channels, as assayed by the rate of channel entry. Thus, it can be concluded that preformed aggregates are not a major source of T domain channel-forming activity.