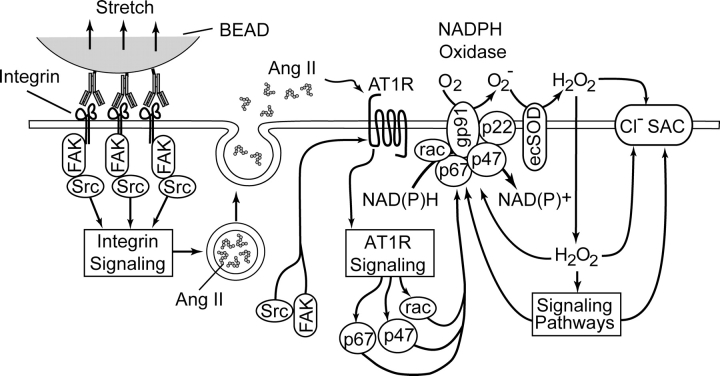
Figure 12.
Simplified proposed model of the mechanotransduction process coupling β1 integrin stretch to activation of Cl− SAC in ventricular myocytes. Integrin stretch triggers the phosphorylation and activation of focal adhesion kinase (FAK) and Src, and the release of Ang II from secretory vesicles. Ang II binds to the AT1 receptor (AT1R) and activates the AT1R signaling cascade. Components of the AT1R signaling cascade, possibly in concert with components of integrin signaling, induce the activation of p47phox, p67phox, and rac, which translocate to the membrane and assemble with gp91phox and p22phox to form the active NADPH oxidase complex. NADPH oxidase recruits NAD(P)H as an electron donor and catalyzes the transmembrane transfer of electrons to molecular O2 to form superoxide (O2 −). Extracellular O2 −· is rapidly converted to membrane-permeant H2O2 by ecSOD. H2O2 may activate Cl− SAC either directly or via ROS-sensitive signaling pathways. The idea that NADPH oxidase may be a closely coupled regulator of the Cl− SAC channel is not illustrated.