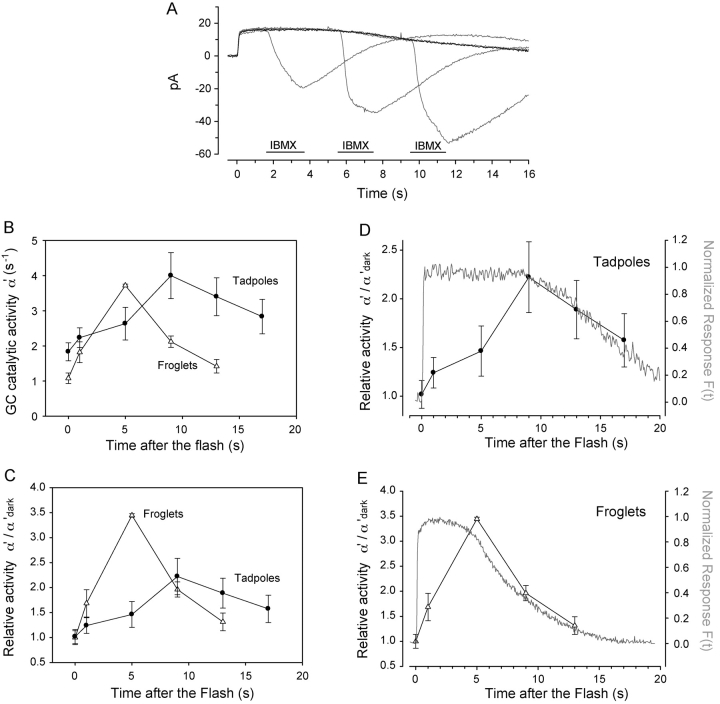
Figure 5.
Guanylate cyclase activity in developing Xenopus rods. (A) Overlay shows currents recorded from a froglet rod in response to brief applications of IBMX at various instances (periods) after stimulation with a saturating flash. Black trace is the control response to 6,000 photoisomerizations. The gray traces are a succession of responses with IBMX applied 1, 5, and 9 s after the flash. Pulse application of IBMX lasted 2 s, concentration was 0.5 mM. (B) Guanylate cyclase activity during the response to a saturating flash was estimated. In froglet rods (triangles, n = 5), resting GC activity (α'dark) was ∼1.1 s−1 and rose rapidly to reach a peak value of 3.8 s−1, 5 s after the flash. In tadpole rods (circles, n = 6), α'dark was 1.6× as active (1.8 s−1), and increased gradually in response to the flash, requiring 9 s to reach its peak activity of ∼4 s−1. (C) Relative guanylate cyclase activity (α'/α'dark) of froglet rods (triangles) increased 3.5-fold in 5 s. α'/α'dark in tadpole rods (circles) grew at a slower rate, increasing only 1.4-fold 5 s after the flash and reaching a peak 2.2-fold increase 9 s after the flash. In that same time (9 s after the flash), α'/α'dark in the froglet rods had already recovered halfway to its resting value. (D and E) The time course of the recovery phase of α'/ α'dark matched closely the recovery of the photocurrents in both tadpole and froglet rods. The shorter time to onset of recovery of the froglet rod response correlates with the fast increase in α'/α'dark activity, while the slower onset of recovery in tadpole rods corresponds to a comparatively sluggish change in α'/α'dark activity. These results suggest that feedback activity mediated by GC strengthens during development to speed up the recovery phase of the responses.