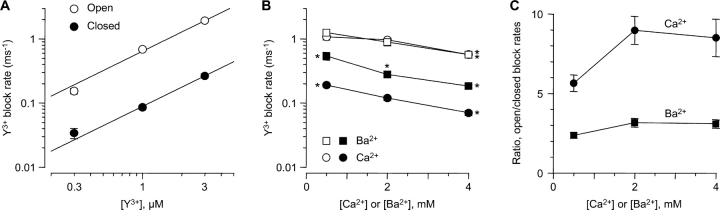
Figure 7.
Comparison of open vs. closed block. (A) Rates of open and closed block at −100 mV in 2 mM Ca2+. Rate constants were calculated as in Fig. 4 C (open block), and as the reciprocal of the fast time constant from the protocol of Fig. 5 (closed block). n = 5–7. The solid lines are fits to bimolecular kinetics, with rate constants of (6.4 ± 0.3) × 108 M−1s−1 (open block) and (0.9 ± 0.1) × 108 M−1s−1 (closed block). (B) Effect of Ca2+ and Ba2+ concentration on open and closed block rates at −100 mV with 1 μM Y3+. Open symbols, open-block rates; solid symbols, closed-block rates. For both open block and closed block, statistically significant differences from the rate at 2 mM Ca2+ are shown by single asterisks (unpaired t tests, all P < 0.01). n = 7–13. (C) The ratio of open- to closed-block rates is higher in Ca2+ than in Ba2+, at all concentrations. At 0.5 mM Ba2+, open block was only 2.4 ± 0.2–fold faster than closed block (n = 9).