Figure 5.
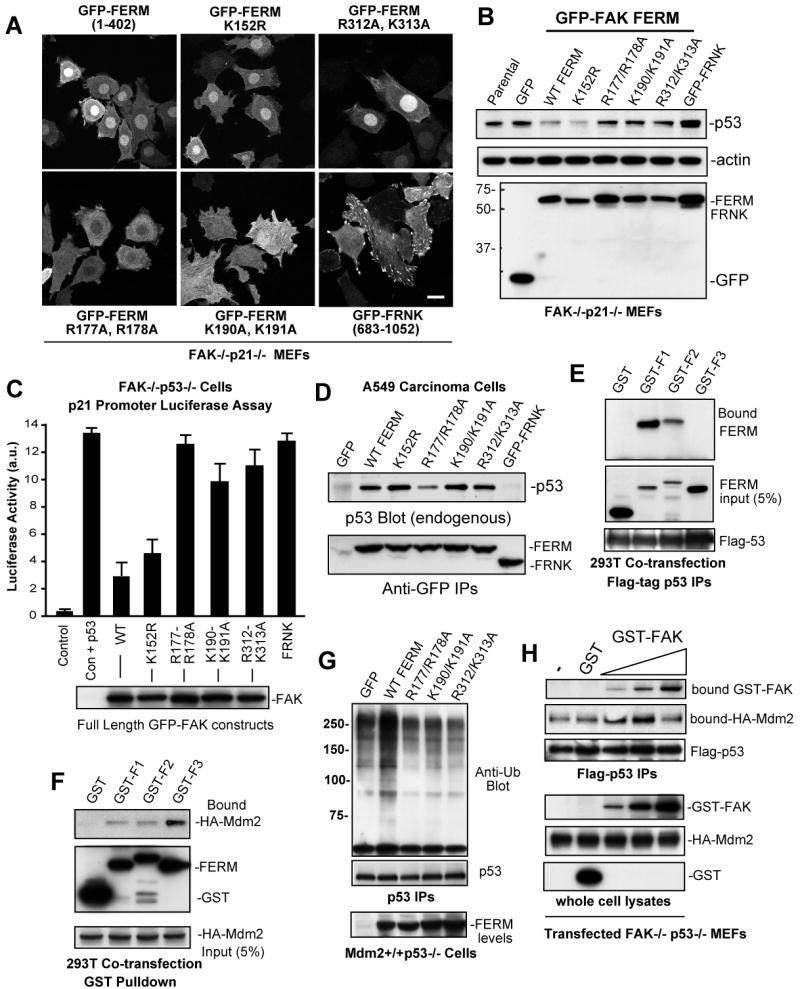
Separate FAK FERM lobes mediate p53 binding, nuclear localization, and Mdm2 association. (A) The indicated GFP-FAK FERM (1-402) constructs or GFP-FRNK were stably-expressed in FAK-/-p21-/- (Pyk2 shRNA) fibroblasts and intracellular distribution visualized by confocal microscopy. Scale bar is 20 μm. (B) Steady-state p53 expression is reduced by FAK FERM expression in FAK-/-p21-/- (Pyk2 shRNA) fibroblasts as detected by p53, actin, and GFP bloting of lysates. (C) FERM domain mutations disrupt full length FAK inhibition of p53 transcriptional activity. FAK-/-p53-/- fibroblasts were transiently-transfected with a 2.4 kb p21 promoter luciferase construct (Vector, V) or in combination with p53 (Vec+p53) and the indicated FAK constructs. Luciferase activity is arbitrary units (a.u.). Values are means of 2 experiments +/- SD. Blotting verified equal FAK construct expression (below). (D) F2 FERM mutations can weaken p53 association. Ad-FAK FERM or FRNK constructs were expressed in A549 cells and association with endogenous p53 analyzed by IP and blotting. (E) FAK FERM F1 lobe binds p53. 293T cells were co-transfected with flag-p53 and the indicated FAK F1, F2, or F3 FERM lobes as GST fusion proteins. Anti-GST blotting of Flag IPs was used to detect FERM lobe association with p53. (F) FAK FERM F3 lobe binds Mdm2. 293T cells were co-transfected with HA-Mdm2 and the indicated FAK F1, F2, or F3 FERM lobes as GST fusion proteins. Cells were treated with MG132 prior to cell lysis (40 μM, 3h), incubated with glutathione agarose, and anti-HA blotting detected bound Mdm2. (G) FAK FERM mutations disrupt FERM-enhanced p53 ubiquitination. Mdm2+/+p53-/- fibroblasts were transfected with flag-p53, transduced with the indicated Ad-FAK FERM constructs, and MG132 was added 3h prior to lysis. p53 IPs were analyzed by anti-ubiquitin, flag-tag, and GFP blotting. (H) Biphasic FAK effects on Mdm2-p53 complex formation. HA-Mdm2 and flag-p53 were transfected into FAK-/-p53-/- fibroblasts, MG132 was added 3h prior to lysis, and GST (500 ng) or increasing amounts of recombinant GST-FAK (10 ng-500 ng) were added prior to p53 isolation by IP. Bound Mdm2 and FAK within a p53 complex were detected by anti-HA and GST blotting.
