Figure 7.
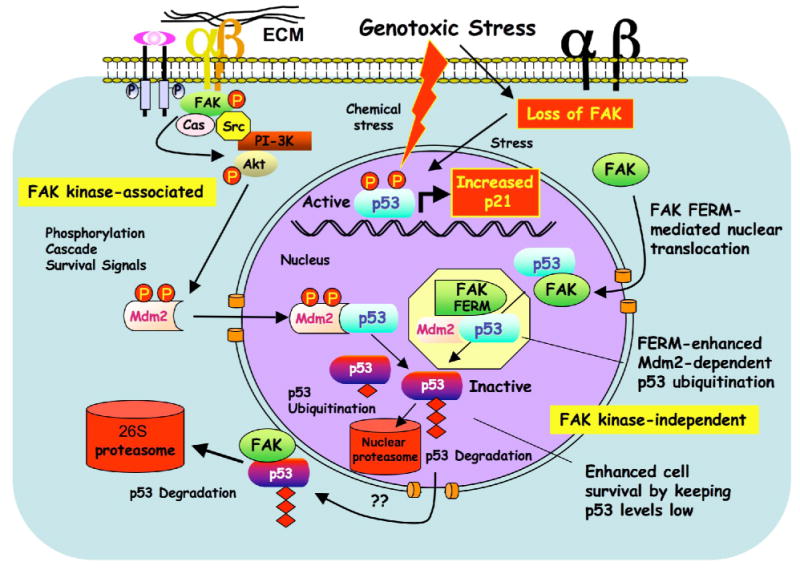
Model of FAK FERM-mediated p53 turnover and cell survival. FAK can function with integrins and growth factor receptors to promote cell survival through signaling cascades such as Akt that can activate ubiquitin E3-ligases such as Mdm2 to maintain low p53 levels. This canonical survival pathway involves FAK kinase activity (left). Under reduced integrin adhesion or conditions of cellular stress, FAK leaves focal contacts sites. This increases the cytoplasmic pool of FAK and enhances FAK nuclear accumulation via FAK-FERM-mediated targeting. Nuclear FAK acts as a scaffold to stabilize a p53-Mdm2 complex, leading to p53 polyubiquitination, and subsequent p53 degradation by nuclear or cytoplasmic proteasomes. This regulatory connection between FAK and p53 is dependent on the FAK FERM domain, but does not require FAK kinase activity (right).
