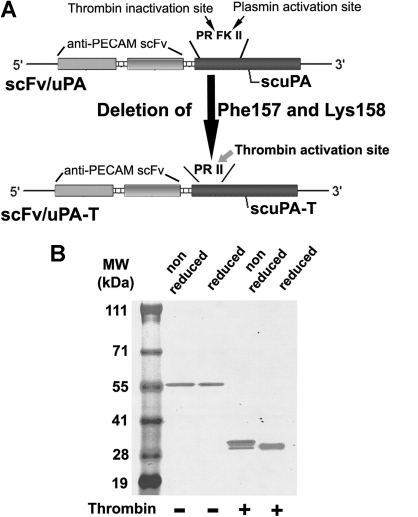
Figure 1.
Molecular design and biochemical characterization of the scFv/uPA-T fusion protein. (A) Single-chain variable fragment (scFv) fused with thrombin-inducible lmw-scuPA (scFv/uPA-T) was generated by deleting Phe157 and Lys158 from the previously described construct, scFv/uPA. This converts the plasmin activation site Pro155-Arg156-Phe157-Lys158-Ile159-Ile160 (PRFKII) into the sequence Pro155-Arg156-Ile157-Ile158 (PRII), which is cleaved by thrombin after Arg156. (B) Migration of the purified fusion protein in the absence or presence of thrombin was analyzed using SDS-PAGE under nonreduced or reduced conditions.