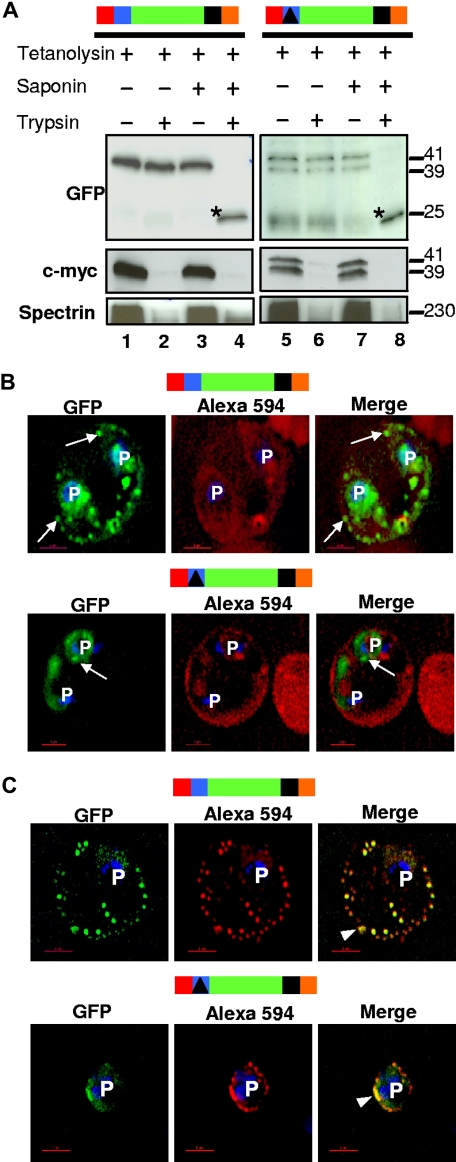
Figure 4.
The HT motif sorts protein into Maurer's clefts without translocation across the PVM. (A) For both HT-GFPmembmyc and Δ-GFPmembmyc, Western blots show protection of GFP but quantitative degradation of myc and erythrocyte spectrin after addition of trypsin to cells where the infected erythrocyte membrane was permeabilized with tetanolysin (lanes 2 and 6). Saponin (which additionally permeabilizes PVM and clefts, lanes 4 and 8) renders GFP susceptible to protease. *, trypsin digested GFP product of 25-kDa. Molecular mass markers are expressed in kilodaltons (kDa). (B) Single optical sections of ghosts resealed with Alexa Fluor 594 anti-GFP antibodies infected with parasites expressing HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel). Cells were viewed live using optics for GFP (green), Alexa Fluor 594/Rhodamine (red), and the merged image is shown in the right panel. Arrows, GFP labeled clefts not labeled with anti-GFP Alexa Fluor 594 conjugate. (C) Immunofluorescence assay of resealed ghosts infected with parasites expressing HT-GFPmembmyc (top panel) or Δ-GFPmembmyc (bottom panel) permeabilized with saponin and treated with anti-GFP Alexa Fluor 594-conjugated antibodies. Images under GFP (green) and Alexa 594 (red) optics and their respective merge are shown. Arrowhead, region of colocalization (in yellow) between GFP and Alexa 594. In all cells, the parasite (p) nuclei were stained with Hoechst 33342 (blue); bar, 2 μm. Schematic representation of the construct is indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) or its replacement (filled triangle in black) fused to GFP (green), transmembrane region (black), and myc (orange).