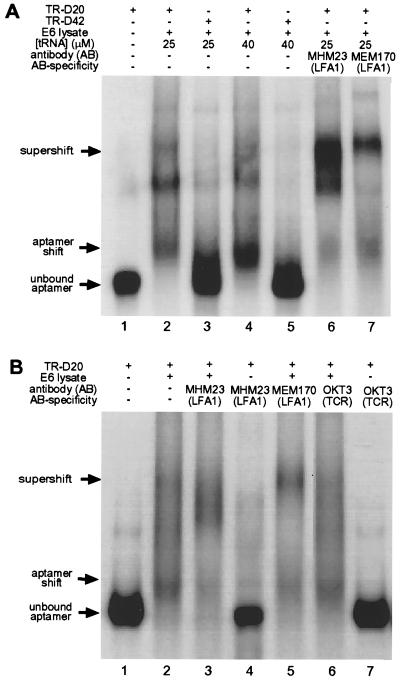
Figure 3.
Gel mobility-shift assays of endogenous CD18 contained in Jurkat E6 cell lysates bound to its cognate aptamer TR-D20. (A) Gel-shift experiment 1. Lane 1, free TR-D20 aptamer RNA; lane 2, shifted band obtained in presence of Jurkat E6 lysate and 25 μM nonspecific competitor tRNA; lane 3, no shift obtained with negative control sequence TR-D42; lane 4, same as lane 2 with 40 μM nonspecific competitor tRNA; lane 5, same as lane 3 with 40 μM nonspecific competitor tRNA; lane 6, specific supershifted band obtained in the presence of antibody MHM23, which recognizes the β2-extracellular domain of LFA-1; lane 7, specific supershifted band obtained in the presence of antibody MEM170, which recognizes the extracellular domain of the αL-subunit of LFA-1. The band pattern may reflect aptamer/integrin complexes of different stoichiometry. (B) Gel-shift experiment 2 with additional controls. All gel-shift experiments were performed in the presence of 30 μM tRNA as a nonspecific competitor. Lane 1, free TR-D20 aptamer RNA; lane 2, shifted band obtained in presence of Jurkat E6 lysate; lane 3, specific supershifted band obtained in the presence of antibody MHM23; lane 4, no shifted aptamer obtained in the absence of Jurkat E6 cell lysate under conditions identical to those in lane 3; lane 5, specific supershifted band obtained in the presence of antibody MEM170; lane 6, no supershift obtained in the presence of antibody OKT3 directed against the T cell receptor. The experimental conditions are otherwise the same as in lane 2; lane 7, no shifted aptamer obtained in the absence of Jurkat E6 cell lysate under conditions identical to those in lane 6.