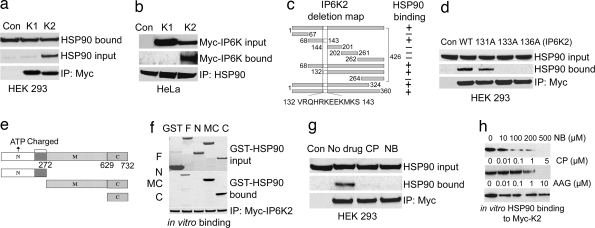
Fig. 2.
HSP90 binds to a specific motif in IP6K2 by its C terminus. (a) Co-IP of endogenous HSP90 by overexpressed Myc-IP6K2 from HEK293 cells. Protein (1 mg) from each cell lysate was immunoprecipitated by anti-Myc antibody and was immunoblotted with monoclonal HSP90 antibody. Lane 1 (Con) shows the Myc-vector control. (b) Co-IP of Myc-IP6K2, not IP6K1, by endogenous HSP90 from HeLa cells. Immunoprecipitation of endogenous HSP90 by monoclonal antibody coprecipitates IP6K2, not IP6K1, as confirmed by blotting with α-Myc antibody. (c) Deletion mapping of IP6K2 to identify HSP90-binding motif. (d) Endogenous HSP90 does not coprecipitate with mutants of IP6K2 in the putative HSP90-binding region. R133A and R136A mutants do not bind, whereas W131A has little effect on binding. (e) Mapping of HSP90 to identify the IP6K2-binding region. Fragments 1–272 [N terminus (N)], 273–732 [middle and C termini (MC)], and 629–732 [C terminus (C)] were generated and cloned into pGEX vector and purified from bacteria. (f) Determination of binding region of HSP90 to IP6K2 by in vitro binding. After incubation of the proteins, the beads were washed, and bound HSP90 was analyzed by blotting with anti-HSP90 monoclonal antibody. (g) IP6K2–HSP90 interaction in cells is disrupted by CP and NB treatment. Overnight drug treatment was followed by immunoprecipitation of Myc-IP6K2 and detection of coprecipitated HSP90 by Western blotting. (h) Binding in vitro of HSP90–IP6K2 is disrupted by CP and NB, but not by AAG. HSP90 bound to Myc-IP6K2 was analyzed by blotting with anti-HSP90 monoclonal antibody.