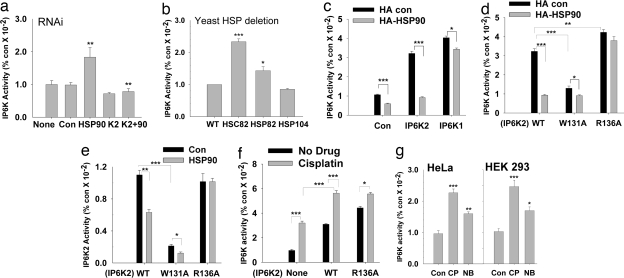
Fig. 3.
HSP90 inhibits IP6K2 catalytic activity. (a) IP6K2 activity in vivo is increased in the absence of endogenous HSP90. HSP90 and IP6K2 (either alone or together) were depleted by using siRNA in [3H]inositol-labeled HEK293 cells and inositol phosphates separated by HPLC. (b) IP6K activity in WT and HSP mutant S. cerevisiae in vivo. IP6 and IP7 were monitored after [3H]inositol labeling of intact cells. (c) HSP90 overexpression leads to decreased IP6K2 activity in vivo. HEK293 cells overexpressing Myc-IP6K1/Myc-IP6K2 either alone or with HA-HSP90 were labeled with [3H]inositol, and inositol phosphates were isolated by HPLC. (d) HSP90 inhibition of IP6K2 in vivo requires binding to IP6K2. IP7 labeled by [3H]inositol was assessed in HEK293 cells cotransfected with Myc-IP6K2 (WT or the mutants) and HA-HSP90. (e) IP6K activity in vitro of WT and mutant IP6K2 in the absence and presence of purified HSP90. HSP90 fails to inhibit IP6K activity of the R136A mutant that does not bind HSP90. IP6K2-W131A is catalytically inactive. (f) CP enhancement of IP7 generation in vivo is influenced by IP6K2–HSP90 binding. IP6K activity of [3H]inositol-labeled HEK293 cells was measured after 30 μM CP treatment overnight. (g) IP6K activity is increased after CP and NB treatment of HEK293 and HeLa cells. After drug treatment, extracts were prepared, and 25 μg of total protein was assayed for IP6K activity.