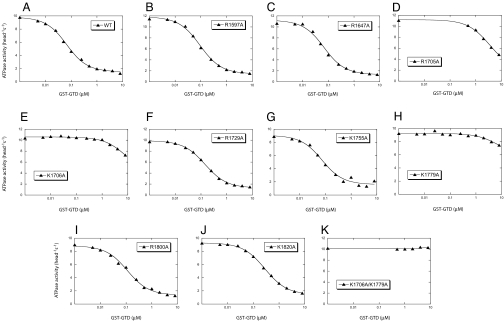
Fig. 5.
Effects of Alanine mutations of conserved basic residues in the GTD on the inhibitory activity of the GST-GTD. The actin-activated ATPase activities of M5HMM in the presence of various concentrations of GST-GTD were measured. The Kd of GST-GTD (except K1705A, K1706A, K1779A, and K1706A/K1779A) to M5HMM were obtained by a quadratic fit, whereas the Kd of GST-GTD mutants (K1705A, K1706A, and K1779A) to M5HMM were obtained by a hyperbolic fit. The calculated values are 0.058 μM (WT), 0.084 μM (R1597A), 0.058 μM (R1647A), 3.611 μM (R1705A), >8 μM (K1706A), 0.134 μM (R1729A), 0.064 μM (K1755A), >8 μM (K1779A), 0.102 μM (R1800A), and 0.271 μM (K1820A).