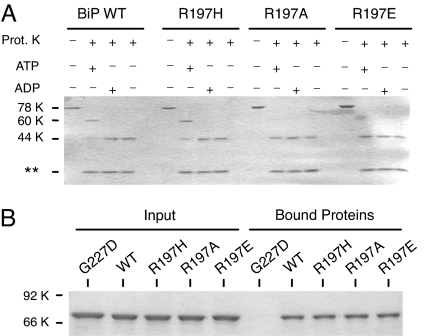
Fig. 2.
Mutations at residue 197 do not affect nucleotide binding but do alter nucleotide-induced conformational changes. (A) Recombinant BiP proteins were incubated in the absence of proteinase K or with proteinase K, along with ATP, ADP, or nucleotide. Samples were analyzed by SDS/PAGE. Full-length proteins migrated at ≈78 kDa, whereas the normal ATP-protected fragment migrated at ≈60 kDa, and the ADP-protected fragment migrated at ≈44 kDa. Proteinase K can be observed at the bottom of the gel. (B) The three R197 mutants, as well as wild-type and G227D BiP, were incubated with ATP–agarose to assess the effects of the mutations on nucleotide binding. The left side of the gel is the input protein, and right side is the protein bound to the ATP–agarose beads. G227D BiP serves as a control for a non-ATP-binding mutant.