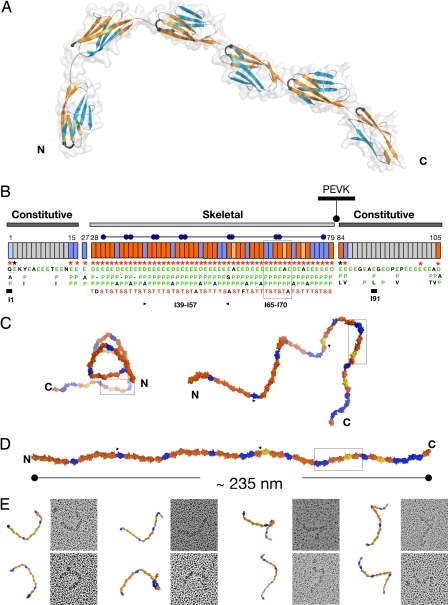
Fig. 1.
Structural order in the poly-Ig from I-band titin. (A) Crystal structure of I65–I70. β-sheets are color coded to emphasize domain torsions. The FG β-hairpin, which claps against the Ig–Ig transition motif EPP, is colored black. (B) Modular composition of the I-band of soleus titin from rabbit (N2A and N2B elements are omitted). Ig domains are represented as boxes, where orange indicates Ig tightly connected and blue represents Ig containing a C-terminal three-residue linker. Annotations refer to conserved features at the Ig–Ig interfaces, where (i) an FG β-hairpin containing an NxxG sequence is marked by red asterisks, (ii) interdomain EPP motifs in green are listed vertically under each domain (Ig exhibiting a natural E-to-A mutation in this motif are colored salmon), and (iii) the conserved S/T residue in the BC loop is shown in red. These features are characteristic of the skeletal but not of the constitutive Ig tandems. Super-repeats of 6 or 10 Ig are indicated by capped bars. Domains with previously known structure are marked with a thick bar. (C) Frontal (Left) and lateral (Right) views of a predicted model of the complete skeletal Ig-tandem in one of its putative slack conformations in solution as calculated from linker arrangements in I65–I70. (D) Model in extended conformation (C and D are color coded as in B). (E) EM images (70 × 70 nm2) of glycerol-sprayed/rotary shadowed I39–I57 accompanied by its corresponding model. The model (fragment indicated by arrows in C) has been oriented to match the micrographs, but no other manipulation has been applied (the 3D conformation of the I39–I57 model can be visualized in SI Movie 2).