Fig. 2.
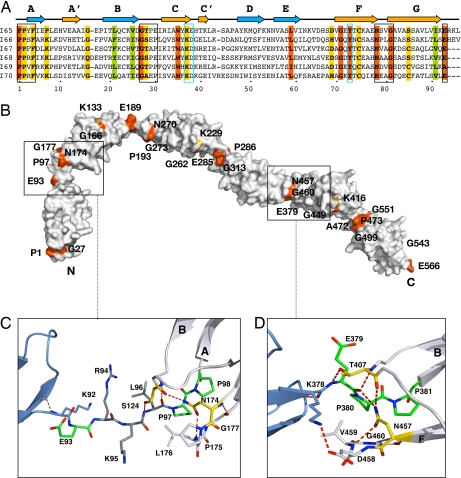
Molecular features of I65–I70. (A) Structure-based sequence alignment where orange and yellow represent >90% and >70% sequence identity, respectively, across all Ig of the skeletal tandem. Green indicates conserved hydrophobic positions. The EPP motif, NxxG sequence in β-hairpin FG, and the BC loop are boxed in black. To ease comparison, the E group in tight linkers is given as the last residue of the preceding domain. A conserved set of residues (KD at the CC′ region and Y at β-strand F) responsible for the conformation of the CC′D loop characteristic of this Ig type is boxed in blue. (B) Molecular surface of I65–I70 colored according to sequence conservation as in A. (C) I65–I66 long linker interface. The three inserted residues are in dark gray. The conserved E is now an integral part of the linker, whereas L, the last of the inserted residues, has replaced it at the N terminus of the following Ig. (D) I68–I69 interface representative of tight connections. The transition motif EPP, an integral part of the N terminus of I69, is in green. The conserved N residue from β-hairpin FG and the T group from the BC loop are in yellow; their interactions are conserved in all Ig constituents of I65–I70. Hydrogen bonds are shown as dashed lines (experimental electron density for both linkers is shown in SI Fig 6).