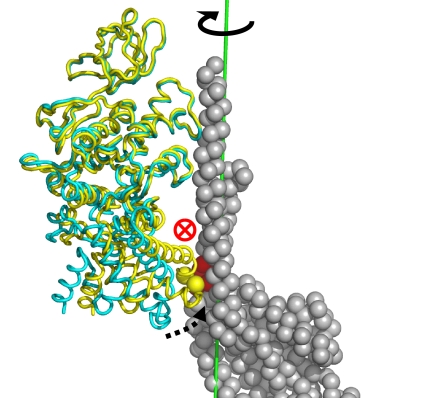
Fig. 4.
Representative snapshot of the torque generation configuration during the 85° substep rotation (see Fig. 3) in the neighborhood of 20°. Only the βE-subunit (yellow ribbon) and γ-subunit (gray spheres) are shown. The β/γ mechanical coupling interaction (βE:I390–L391 and γ:20–25) identified from the simulation is highlighted by spheres (β, yellow; γ, red). The initial conformation of the βE-subunit (fully open, before ATP binding) is shown as a cyan ribbon. During the ATP binding, the helix–turn–helix-motif domain of the βE-subunit swings up by ≈30° (represented by a dotted black arrow). This conformational change results in a close contact between βE:I390–L391 and the N-terminal helix of the γ-coiled-coil at γ:20–25 (most likely through hydrophobic interactions). The βE movement yields an off-axis force that pushes the N-terminal helix of the γ-stalk in a direction into the plane of the paper (indicated by the red ⊗). This results in a unidirectional γ-stalk rotation (indicated by the solid black arrow around the green rotation axis). The illustrations were made with the program PyMOL.