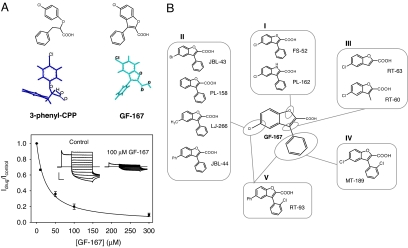
Fig. 2.
Effect of rationally designed molecules on ClC-Ka. (A) Insertion of the chlorophenoxy moiety of 3-phenyl-CPP into a benzofuran system. The chemical structure and the lowest energy conformation of each molecule are shown. Dose–response relationship of the block at 60 mV by GF-167. The line is drawn according to the equation reported in Methods. Voltage–clamp traces of ClC-Ka currents before and after application of 100 μM of GF-167 are shown in Inset. (Horizontal scale bar, 50 ms; vertical scale bar, 5 μA.) (B) Chemical structure of GF-167 derivatives. I, compounds with isosteric substitutions of the oxygen atom of the benzofuran nucleus; II, compounds with different substituents on the benzofuran nucleus; III, compounds with elimination of the phenyl group; IV, compound with a chlorine atom on the phenyl group; V, compound with an additional phenyl group on the benzofuran nucleus and a chlorine atom on the phenyl group.