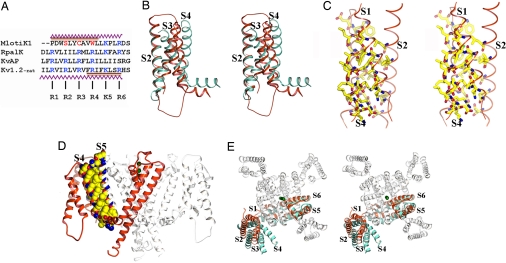
Fig. 4.
The S1–S4 domain. (A) Structure-based sequence alignment of S4 regions from the MlotiK1 (GI:14023393), RpalK (GI:39937293), KvAP (GI:30749950), and Kv1.2 (GI:73536154) channels. Conserved positively charged residues are shown in blue. MlotiK1 residues corresponding to R2, R3, and R4 are shown in red. The zigzag lines indicate residues in the S4 helices of MlotiK1 and Kv1.2. The brown bar indicates regions of S4 adopting 310 conformation in MlotiK1 and Kv1.2. (B) Stereoview of superposition of S1–S4 domains from MlotiK1 (red) and Kv1.2 (cyan) via S2 and S1. TMs are labeled. (C) Stereoview of residues within the core of S1–S4 domain. TMs are labeled. (D) Side view of MlotiK1. The extracellular side of the membrane is above the molecule. One subunit is shown in red. The residues involved in the interaction between S4 and S5 are shown in Corey–Pauling–Koltun representation. (E) Stereoview from the extracellular side. The MlotiK1 channel (in white and red) and a Kv1.2 subunit (in cyan) are superposed via the pore region. TMs are labeled. The green spheres represent K+.