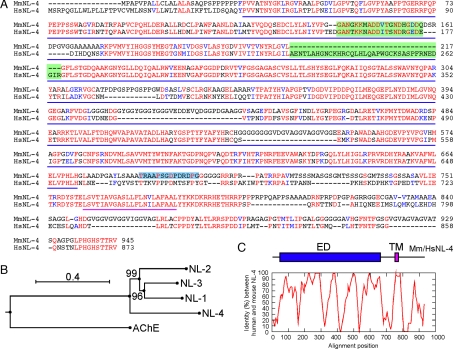
Fig. 1.
The mouse NL-4 protein. (A) Alignment between mouse and human NL-4 protein sequences. All specific NL signatures, including the signal peptide, the esterase domain (underlined in blue), the transmembrane domain (underlined in purple), and the C-terminal PDZ domain-binding motif are conserved in mouse NL-4. Identical and similar residues are printed red and blue, respectively. Amino acids on green background correspond to alternatively spliced exons in human NL-4. Amino acids on blue background represent the peptide used to generate anti-NL-4 antibodies (SI Fig. 5). (B) Phylogenetic tree of the mouse NLs, constructed using the neighbor joining method. The tree has been rooted using the mouse sequence of acetylcholine esterase (AChE). Values at the bifurcation points are bootstraps for 100 samplings. The scale bar indicates 0.4 substitutions per site. (C) Pairwise alignment of human and mouse NL-4. Percentage identity between human and mouse NL-4 protein was calculated using 21-aa sliding windows (ED, esterase domain; TM, transmembrane domain).