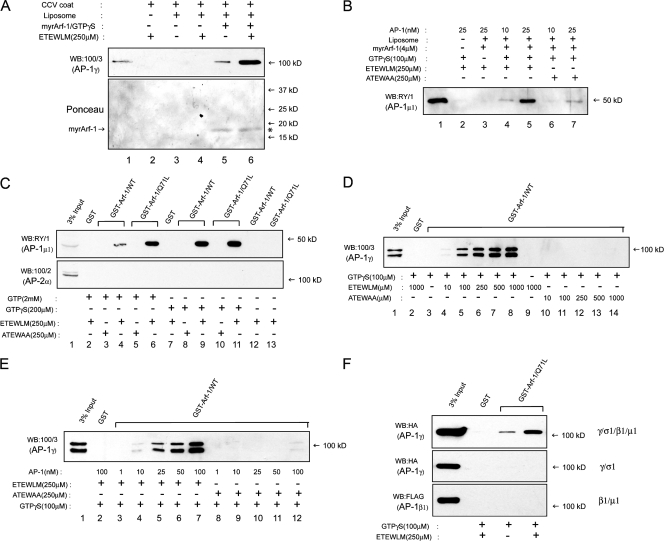
Figure 1.
Binding of the CI-MPR ETEWLM peptide to AP-1 stimulates its association with activated Arf-1. (A) Recruitment of CCV-derived AP-1 to soybean liposomes is greatly enhanced only in the presence of 250 μM ETEWLM peptide (top, compare lanes 5 and 6) even though similar amounts of activated Arf-1 are found on the liposomes, as seen in the Ponceau stain of the blot (bottom, *). Lane 1 represents 5% of the CCV coat fraction input. (B) Recruitment of 25 nM AP-1 purified from BAC to liposomes is stimulated by the ETEWLM peptide (lanes 5 vs. 7) in a GTPγS-dependent manner (lanes 3 vs. 5). The reactions contained 4 μM of myristoylated Arf-1, 100 μM GTPγS, and the indicated concentrations of AP-1. Lane 1 represents 20% of the input from the reaction containing 25 nM AP-1. (C) Pulldown of AP-1 from BBC with both WT GST–Arf-1 and the Q71L mutant preloaded with 2mM GTP or 100 μM GTPγS is dependent on the presence of the ETEWLM peptide. No binding to AP-2 was detected under these conditions. (D and E) Binding of AP-1, purified from BAC, to GST–Arf-1 is a function of the concentration of the ETEWLM peptide (D) and purified adaptor (E). The AP-1 concentration was kept constant at 25 nM in D, whereas the peptide concentrations were kept constant at 250 μM in E. The doublet observed on the blots in D and E, and in subsequent experiments where BAC served as the source of cytosolic AP-1, is the consequence of partial proteolysis of the appendage domain of the γ subunit of AP-1 in our adrenal cytosol preparations. The γ subunit of AP-1 in our bovine brain CCV coat preparations did not undergo such proteolysis, as shown in A. (F) The dileucine peptide–dependent binding of AP-1 to activated GST–Arf-1 requires the tetramer because neither hemicomplex bound.