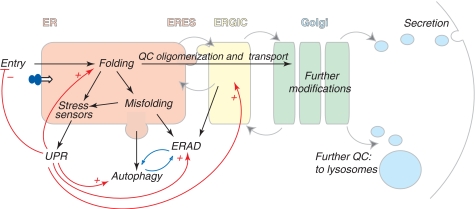
Figure 1.
The early secretory pathway. Proteins destined to the extracellular space or to organelles of the secretory route are synthesized by ER-bound ribosomes and cotranslationally translocated (entry) into the ER. Here they attain their native structure (folding), under strict QC scrutiny. Only properly folded and assembled proteins can reach the Golgi, where they are further modified, to be transported to the extracellular space or to lysosomes. Gray arrows indicate the direction of vesicles moving among different compartments; dark arrows indicate the pathways followed by cargoes in the early secretory pathway; red lines show homeostatic control pathways (+ stimulatory, − inhibitory). Misfolded proteins are recognized, retained and eventually routed to degradation by ERAD or autophagy (which are likely reciprocally regulated, as indicated by the blue arrows). Some misfolded soluble ERAD substrates are transported to ERGIC or cis-Golgi before retrotranslocation and degradation. Too high load for the folding machinery or the accumulation of misfolded proteins activate resident ER stress sensors, which elicit the UPR. ER stress can selectively inhibit protein entry into the ER, and increase the capacity of folding and degradation (via ERAD and autophagy). The UPR induces also molecules acting downstream of the ER.