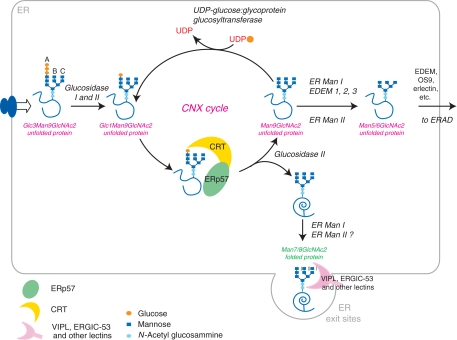
Figure 2.
The CNX/CRT cycle. After transfer of the preformed core oligosaccharide (Glc3Man9GlcNAc2) onto nascent proteins, glucosidase I and II sequentially remove the two terminal glucoses from the A branch. The monoglucosylated Glc1Man9GlcNAc2 unfolded protein can now interact with the lectin chaperones CNX and CRT. In association with the oxidoreductase ERp57, CNX and CRT prevent aggregation and facilitate glycoprotein folding. Removal of the glucose by glucosidase II (Man9GlcNAc2) interrupts the interaction of the protein with CNX/CRT. If the protein has attained its native structure, it can now proceed along the secretory pathway by bulk flow or by interaction with specific lectin transporters such as ERGIC-53 or VIPL. If unfolding persists, the glycoprotein is recognized by UGGT1, which places a single glucose back onto the A branch, causing the protein to enter the CNX/CRT cycle again. Mannose trimming causes exit from the CNX/CRT cycle. Misfolded proteins can be recognized by specific lectins (EDEMs, OS9, etc) and targeted to degradation.