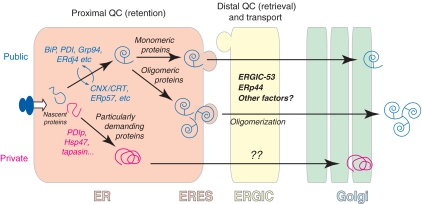
Figure 3.
QC in the early secretory pathway. Chaperones and folding assistants can be grouped in different classes according to their specificity and subcellular localization. The majority of secretory proteins utilize public chaperones: some initially go with BiP, PDI and their partners, others enter the CNX/CRT cycle, the choice depending on the location of the N-glycans. Certain proteins that are produced in large amounts, or are intrinsically difficult to fold, are assisted by specific (private) chaperones and enzymes (see also Box 2). In addition, QC can occur in sequential steps. After a proximal QC, certain substrates (generally multimeric proteins) seem to undergo also distal QC checkpoints in ERGIC and cis-Golgi. This model could mediate cargo concentration and selective export of oligomerized species, thus coupling fidelity and efficiency in the secretory protein factory. While proximal QC can rely on simple retention, the distal checkpoints likely imply substrate retrieval to the ER, either for further attempts to fold, or for retrotranslocation and degradation.