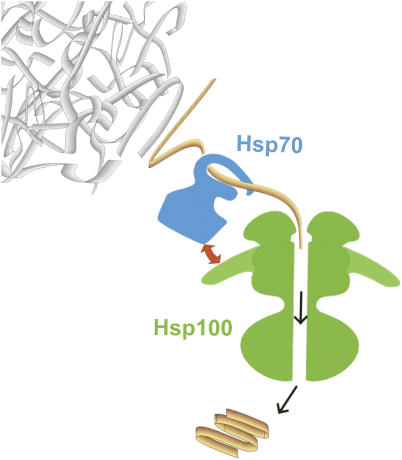
Figure 1.
Model for the mechanism of action of Hsp70 and Hsp100 chaperone system in disaggregation of protein aggregates. First, the Hsp70 chaperone system disentangles the polypeptides from aggregates. The polypeptides are then transferred to ClpB/Hsp104 and unfolded by translocation through the central channel, driven by energy from ATP hydrolysis. The unfolded polypeptides are released following translocation and refold either spontaneously or with the assistance of chaperones. The Hsp70 chaperone system and ClpB/Hsp104 cooperate in the disaggregation process in a specific way (marked with the red arrow). The interaction is linked to the propeller-shaped middle domain of ClpB.