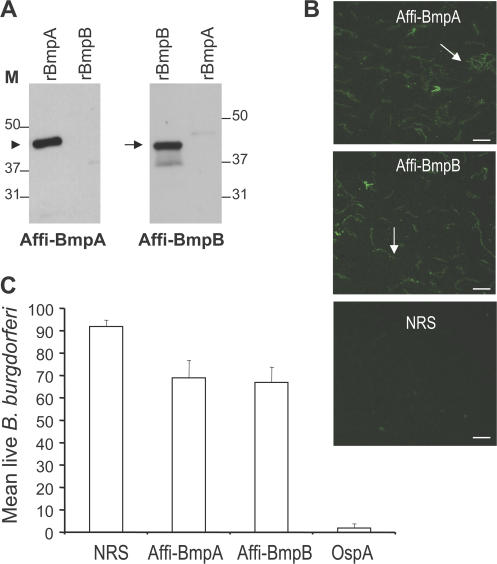
Figure 2.
Characterization of affinity-purified BmpA and BmpB antibodies. (A) 1 ng of recombinant Bmp proteins (rBmpA and rBmpB) was probed with either affinity-purified BmpA (Affi-BmpA) or BmpB (Affi-BmpB) antibodies. The arrowhead and arrow indicate the positions of BmpA and BmpB, respectively. (B) BmpA or BmpB antibodies bind the surface of intact unfixed B. burgdorferi (arrows). Spirochetes were immobilized on glass slides and probed with affinity-purified BmpA (Affi-BmpA), BmpB (Affi-BmpB) antibodies, or normal rabbit sera (NRS). Images were acquired using the 40× objective lens of an epifluorescence microscope (Axiovert 200M; Carl Zeiss, Inc.). Bars, 10 μm. (C) Borreliacidal activities of BmpA or BmpB antibodies in vitro. Spirochetes were incubated with normal rabbit sera (NRS), affinity-purified BmpA (Affi-BmpA), BmpB (Affi-BmpB), or rabbit OspA antibodies (OspA). The number of viable spirochetes was assessed by fluorescent microscopy after labeling the spirochete preparations with vital dyes at 48 h of incubation. Data represent the mean number of viable spirochetes/microscopic field after antibody incubation ± the SEM from three independent experiments. The number of viable spirochetes exposed to the affinity-purified BmpA or BmpB antibodies were significantly diminished compared with spirochetes that received NRS. n = 3. p < 0.01. As expected, OspA antisera resulted in the complete killing of B. burgdorferi.