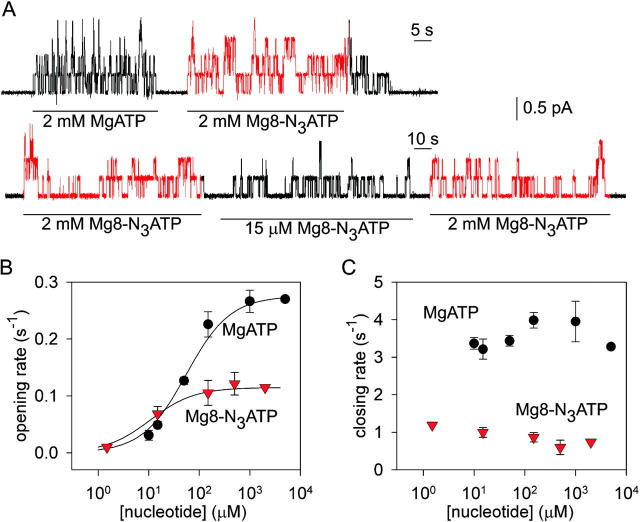
Figure 1.
The 8-azido group slows CFTR Cl− channel opening and closing. (A) Outward currents (at +40 mV) activated by nucleotides indicated beneath traces in patches excised from oocytes expressing WT CFTR, after prior exposure to PKA plus 5 mM MgATP. (B and C) Mean rates of channel opening to a burst (rCO, B) and of closing from a burst (rOC, C) vs. [MgATP] (filled circles) or [Mg8-N3ATP] (red triangles), from analysis of records as in A (lower trace). For each test concentration (e.g., 15 μM Mg8-N3ATP, in A), kinetic parameters were normalized to the average of the values obtained from the same channels during the bracketing exposures to saturating nucleotide (e.g., 2 mM Mg8-N3ATP, in A). These ratios were then scaled by our estimates of absolute opening and closing rates at saturating [nucleotide] (see materials and methods): for MgATP, rCO(5 mM MgATP) = 0.27 ± 0.03 s−1 and rOC(5 mM MgATP)= 3.3 ± 0.2 s−1 (n = 18; Vergani et al., 2003). For Mg8-N3ATP we multiplied those rates by the factors (0.42 for rCO, and 0.19 for rOC) determined by direct comparison (as in A, top trace), assuming that 2 and 5 mM MgATP are both saturating concentrations. Curves in B show Michelis-Menten fits, with parameters (mean ± SEM; 2 ≤ n ≤ 7) K0.5 = 55 ± 5 μM and 11 ± 3 μM and Vmax = 1.02 ± 0.02 and 1.01 ± 0.04, for MgATP and Mg8-N3ATP, respectively. The high apparent affinity for Mg8-N3ATP is directly evident in Fig. 1 A, bottom trace: thus, rCO at 15 μM Mg8-N3ATP averaged 60 ± 11% (n = 7) of rCO at saturating, 2 mM, Mg8-N3ATP, whereas rCO at 15 μM MgATP was only 17 ± 1% (n = 8) of rCO at saturating [MgATP] (Fig. 1 B).