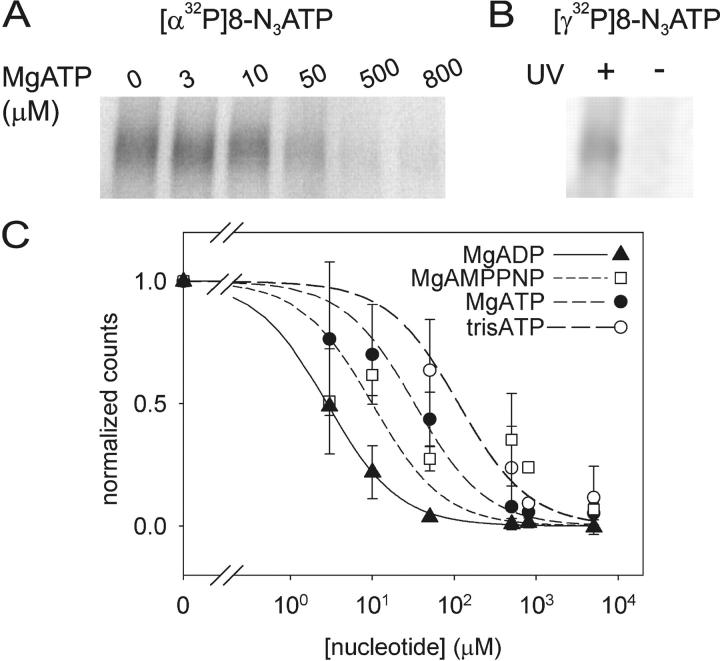
Figure 2.
[α32P]8-N3ATP and [γ32P]8-N3ATP binding to Flag-CFTR in HEK293T cell membranes and inhibition by nucleotides. (A) Autoradiogram showing competition by cold MgATP for binding of 5 μM [α32P]8-N3ATP to CFTR at 0°C, followed by UV irradiation, washing, immunoprecipitation, and SDS-PAGE. (B) CFTR incubated with 5 μM [γ32P]8-N3ATP at 0°C was photolabeled only after UV irradiation, indicating lack of phosphorylation by endogenous kinases under these conditions. (C) Quantification of competition for binding of 5 μM [α32P]8-N3ATP by unlabeled MgATP (as in A), MgADP, or MgAMPPNP (all in Mg2+-containing buffer), or by unlabeled TrisATP (in Mg2+-free buffer). Photolabeling was normalized to the signal obtained in the absence of competing nucleotide (note broken abscissa), and the data (error bars show ±SD) fitted assuming simple competition and a Kd of 5 μM for Mg8-N3ATP, or 8 μM for free 8-N3ATP. Ki values from the fits were 1 ± 1 μM for MgADP (filled triangles, n = 2), 6 ± 2 μM for MgAMPPNP (empty squares, n = 4), 16 ± 4 μM for MgATP (filled circles, n = 4), and 69 ± 20 μM for TrisATP (empty circles, n = 3). Given the finite duration of UV irradiation, these apparent binding affinities might be less accurate than the ratios of the values obtained for the different nucleotides.