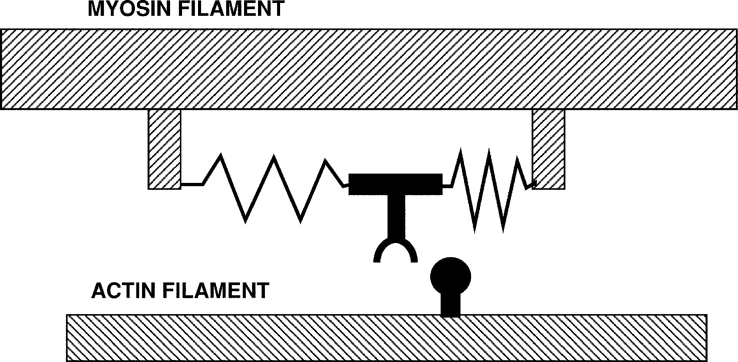
Figure 6.
The first model of a motor protein, proposed by Sir Andrew Huxley in 1957, before the myosin cross-bridge had been visualized. The myosin is shown connected to the filament backbone by two springs. As shown, a thermal fluctuation has moved the myosin part of the way toward the actin site, which is entering from the right. A further fluctuation can carry it the rest of the distance, where binding to actin traps the springs in distorted positions. Relaxation of the springs then drags the actin to the left. When the actin myosin positions reach the center, where both springs are in their relaxed positions, rapid binding of ATP releases the myosin from actin. A similar cyclic interaction between motors and polymers, orchestrated by the binding of nucleotides is thought to produce the motility of both actin-myosin and kinesin-microtubule motor systems.