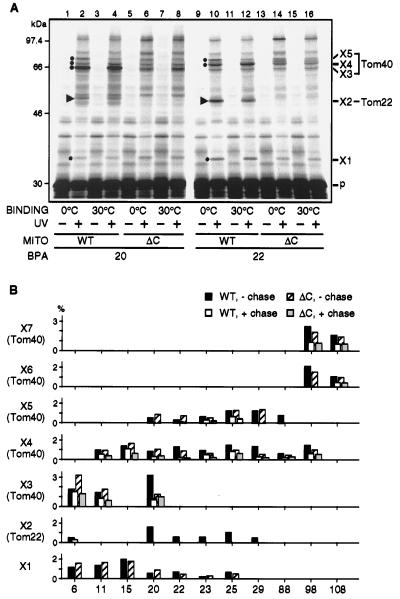
Figure 3.
Effects of deletion of the IMS domain of Tom22 on the crosslinking between pSu9-DHFR and the TOM components. (A) Mitochondria were prepared from the yeast MNMS-MAS17 strain (WT) or MNMS-MAS17Δ120–152 strain (ΔC). pSu9-DHFR containing BPA at position 20 or 22 was bound to CCCP-treated mitochondria at 0°C (lanes 1, 2, 5, 6, 9, 10, 13, and 14) or at 30°C (lanes 3, 4, 7, 8, 11, 12, 15, and 16). After binding, the mitochondria were isolated by centrifugation and subjected to UV irradiation for 5 min at 0°C (even-numbered lanes). UV, UV irradiation; MITO, mitochondria; BPA, positions at which BPA was introduced; p, pSu9-DHFR. Triangles indicate the crosslinked product X2 involving Tom22. Dots indicate the crosslinked products X1, X3, X4, and X5. (B) pSu9-DHFR containing BPA at positions 6, 11, 15, 20, 22, 23, 25, 29, 88, 98, and 108 was bound to CCCP-treated mitochondria prepared from MNMS-MAS17 (WT) or from MNMS-MAS17Δ120–152 (ΔC) at 30°C. The samples were diluted with MSC buffer containing 10 mM KCl and divided into halves, and the mitochondria were reisolated by centrifugation. One aliquot was resuspended with MSC buffer containing 10 mM KCl and kept on ice (− chase). The other aliquot was resuspended with chase buffer and incubated for 10 min at 30°C (+ chase). The samples were divided into halves and one aliquot was subjected to UV irradiation for 5 min at 0°C. Proteins in all samples were analyzed by SDS/PAGE, and yields of crosslinked products X1–X7 were quantified as described in Fig. 2.