Fig. 4.
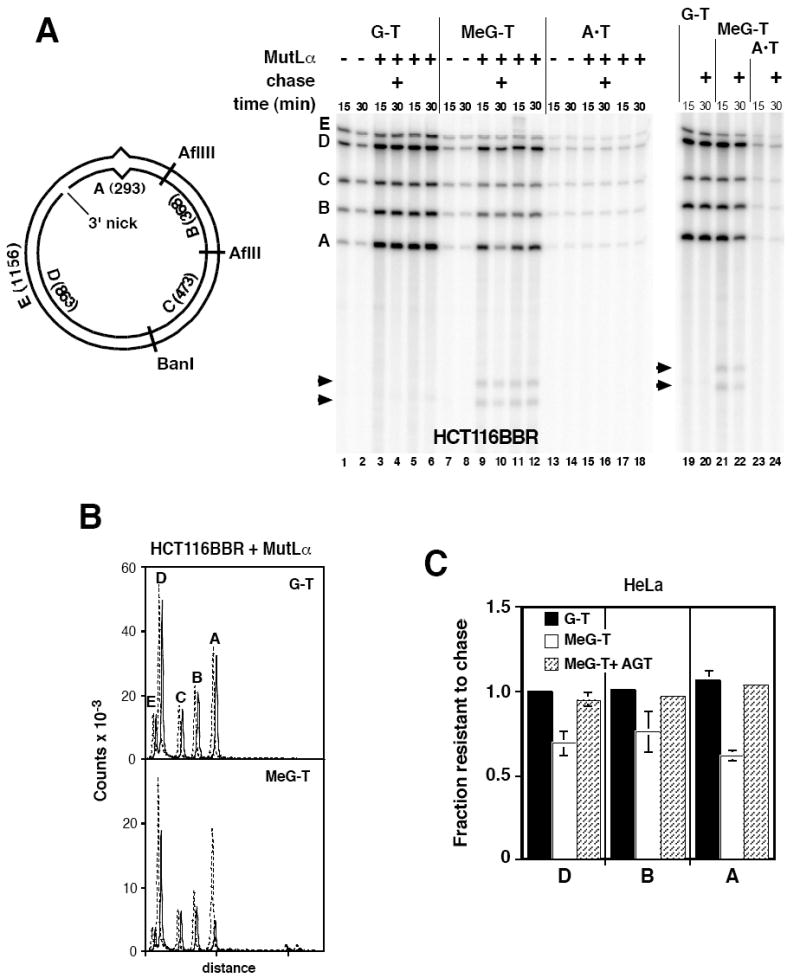
Iterative excision occurs on a 3’ MeG-T irreparable heteroduplex. (A) Nuclear extracts from HCT116BBR or O6BG-treated HeLa cells were incubated with 3’ substrates and dNTPs including [α-32P]dATP under label-chase conditions (Materials and Methods) in the absence or presence of exogenous 25 nM MutLα. After 15 min, a sample was removed and quenched. The remainder of the reaction was supplemented with excess cold dATP and incubation continued for an additional 15 min prior to termination (30 min sample). After cleavage with AflII, AflIII, and BanI, and nicking with N.BbvCIA, recovered DNA was analyzed on polyacrylamide gel in the presence of 7 M urea. Identity and sizes of DNA fragments are shown in the diagram. Note that fragment E is derived exclusively from the continuous heteroduplex strand, while fragments A and D are derived solely from the incised strand. Arrowheads indicate two novel fragments produced on the 3’ MeG-T heteroduplex. (B) Quantitative phosphorimager label-chase results obtained with MutLα-supplemented HCT116BBR extract are shown. These traces correspond to lanes 3, 4, 9, and 10 of panel A. Dashed (15 min label) and solid lines (15 min label, 15 min chase) are offset for presentation. (C) Quantitative results are shown for label-chase experiments performed in HeLa nuclear extract. Normalized specific activities were calculated relative to that of fragment C, and the fractional specific activity resistant to chase calculated. Because fragment C was used to correct for variation in DNA loading, the value of this parameter for fragment C was 1 in all cases. Values shown are the average (± one standard deviation) for three determinations for 3’G-T and 3’ MeG-T heteroduplexes. Results for AGT-treated 3’ MeG-T DNA are the mean of two determinations, with range of these two values indicated.
