Abstract
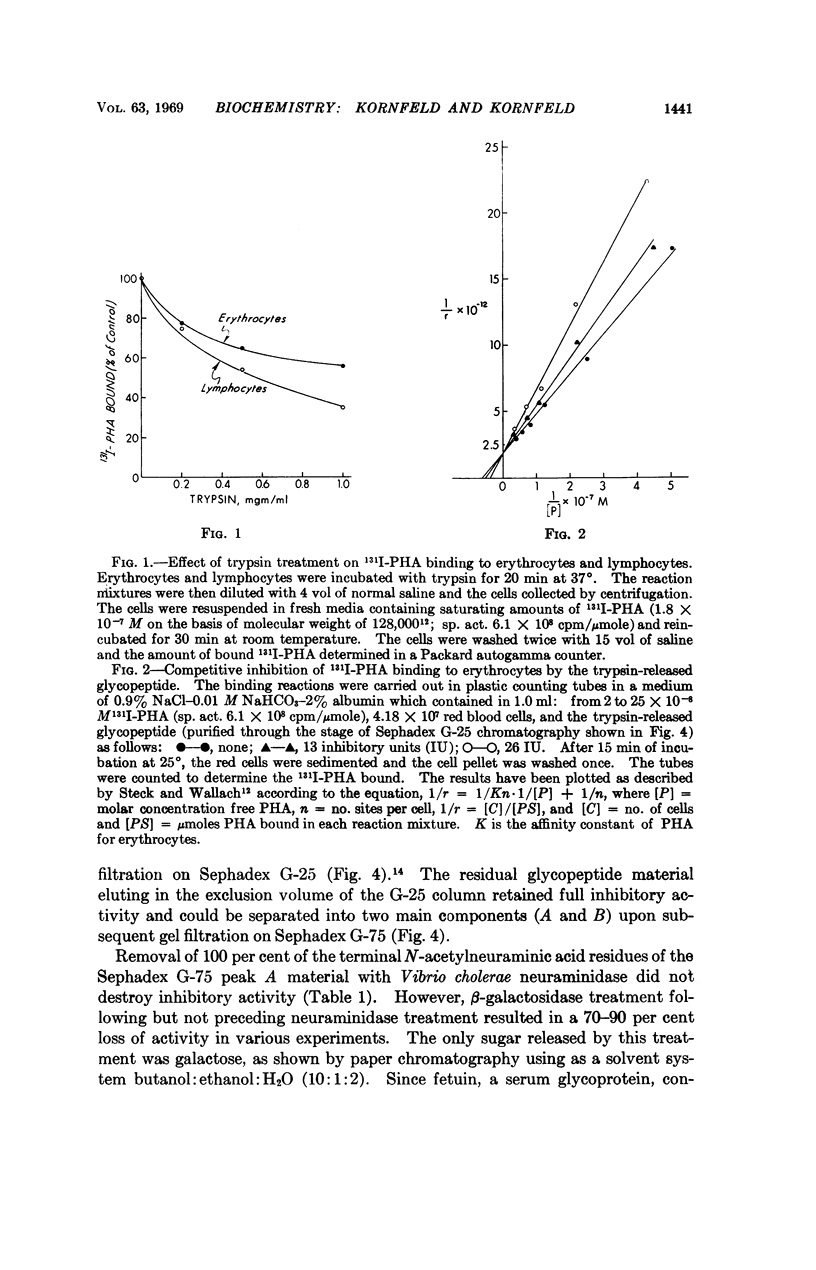
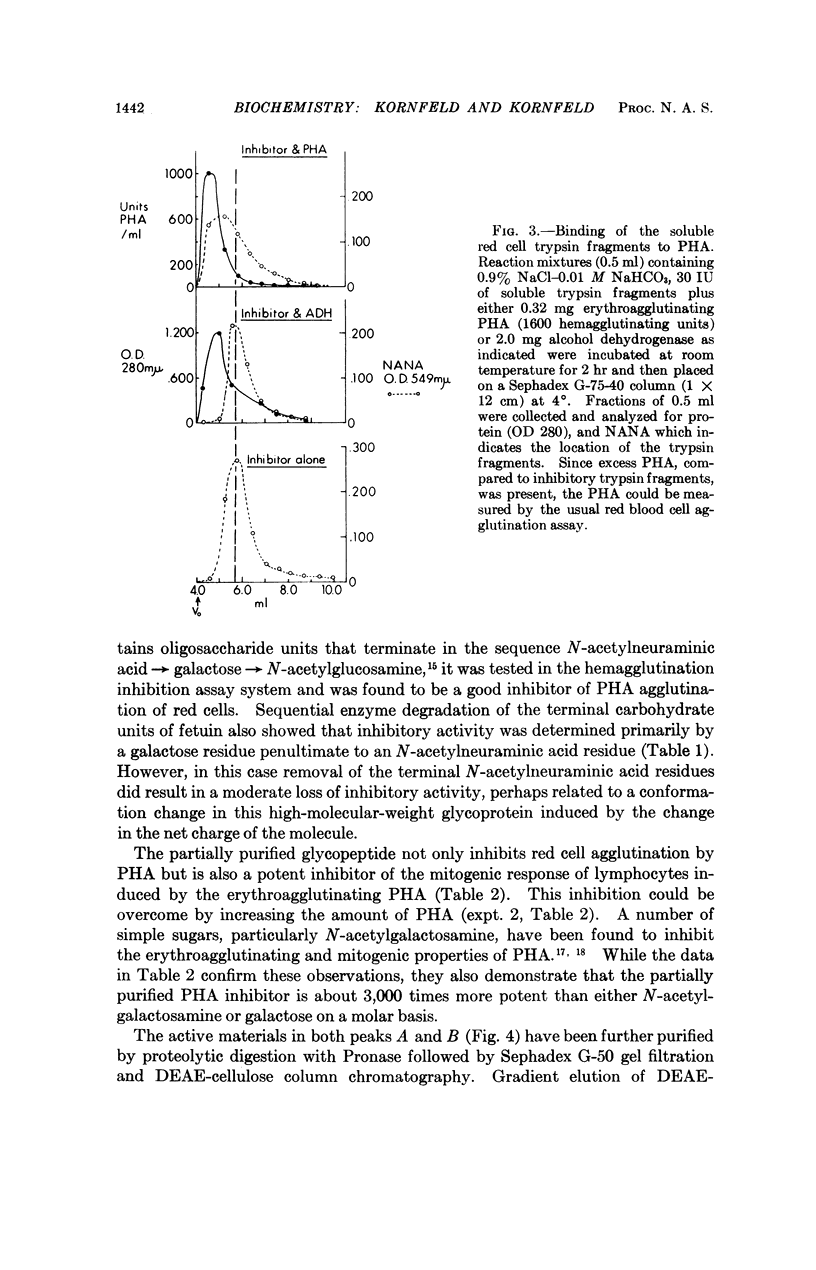
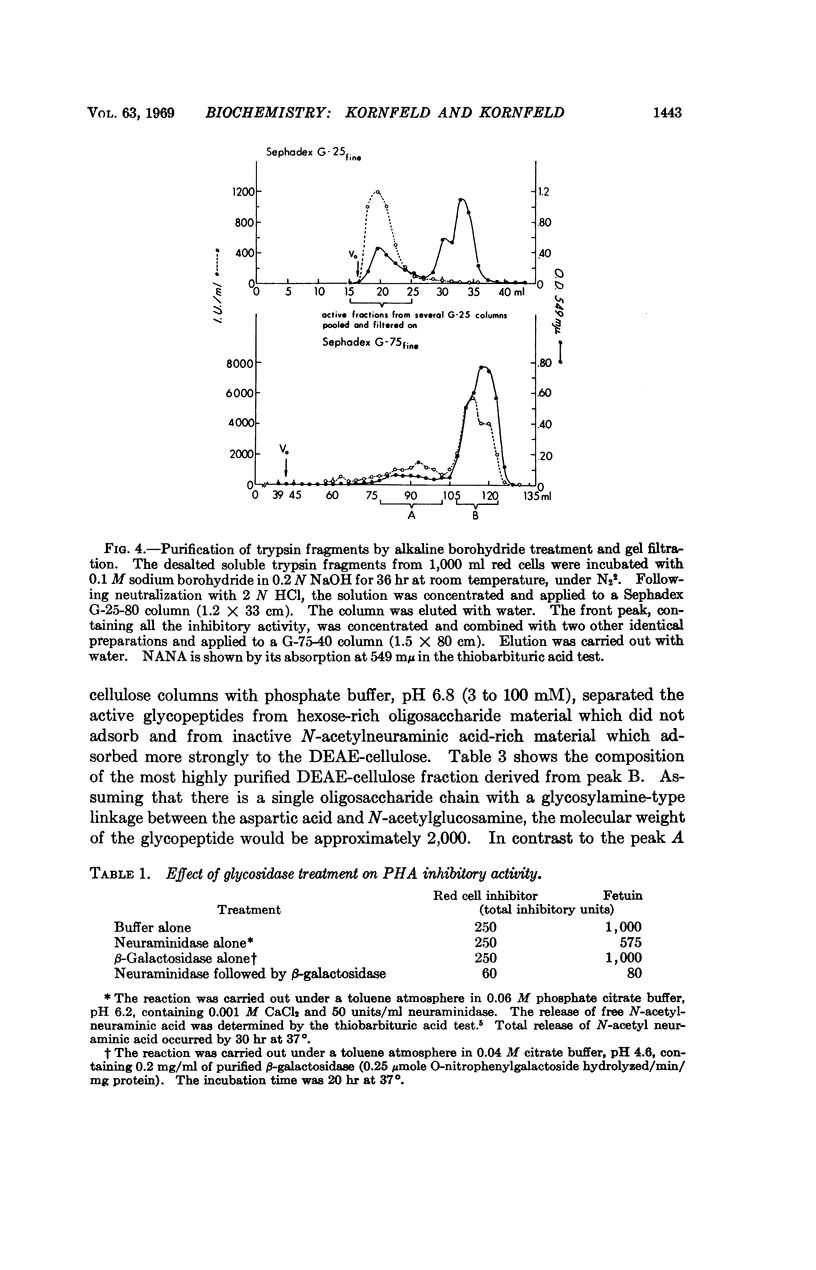
Trypsin treatment of human erythrocytes releases a soluble glycopeptide which binds to phytohemagglutinin and abolishes the erythroagglutinating and lymphocyte-stimulating properties of this molecule. The glycopeptide has been purified by alkaline borohydride treatment, proteolytic digestion, gel filtration, and DEAE-cellulose chromatography. The most highly purified glycopeptide has a molecular weight of about 2,000. The specificity for binding to phytohemagglutinin resides in the oligosaccharide portion of the molecule with the determinant sugar being a galactose residue which is penultimate to a N-acetylneuraminic acid in some chains and uncovered in others. The glycopeptide is about 3,000 times more potent than either N-acetylgalactosamine or galactose in inhibiting the mitogenic response of lymphocytes induced by phytohemagglutinin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- AWAI M., BROWN E. B. Studies of the metabolism of I-131-labeled human transferrin. J Lab Clin Med. 1963 Mar;61:363–396. [PubMed] [Google Scholar]
- Berberg H., Woodruff J., Hirschhorn R., Gesner B., Miescher P., Silber R. Phytohemagglutinin: inhibition of the agglutinating activity by N-acetyl-D-galactosamine. Science. 1966 Nov 25;154(3752):1019–1020. doi: 10.1126/science.154.3752.1019. [DOI] [PubMed] [Google Scholar]
- Borberg H., Yesner I., Gesner B., Silber R. The effect of N acetyl C-galactosamine and other sugars on the mitogenic activity and attachment of PHA to tonsil cells. Blood. 1968 Jun;31(6):747–757. [PubMed] [Google Scholar]
- COOK G. M., HEARD D. H., SEAMAN G. V. A sialomucopeptide liberated by trypsin from the human erythrocyte. Nature. 1960 Dec 17;188:1011–1012. doi: 10.1038/1881011a0. [DOI] [PubMed] [Google Scholar]
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li Y. T. Studies on the glycosidases in jack bean meal. I. Isolation and properties of alpha-mannosidase. J Biol Chem. 1967 Dec 10;242(23):5474–5480. [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A. Immunochemical studies on blood groups. XLI. Proposed structures for the carbohydrate portions of blood group A, B, H, Lewis-a, and Lewis-b substances. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1470–1477. doi: 10.1073/pnas.61.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Ikemoto S. A sialoglycopeptide liberated from human red cells by treatment with trypsin. Nature. 1966 Oct 8;212(5058):198–199. doi: 10.1038/212198a0. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- SEAMAN G. V., HEARD D. H. The surface of the washed human erythrocyte as a polyanion. J Gen Physiol. 1960 Nov;44:251–268. doi: 10.1085/jgp.44.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. Studies on the monosaccharide sequence of the serum glycoprotein fetuin. J Biol Chem. 1962 Mar;237:646–652. [PubMed] [Google Scholar]
- STECK T. L., HOELZLWALLACH D. F. THE BINDING OF KIDNEY-BEAN PHYTOHEMAGGLUTININ BY EHRLICH ASCITES CARCINOMA. Biochim Biophys Acta. 1965 Mar 8;97:510–522. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Watkins W. M. Blood-group substances. Science. 1966 Apr 8;152(3719):172–181. doi: 10.1126/science.152.3719.172. [DOI] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Winzler R. J., Harris E. D., Pekas D. J., Johnson C. A., Weber P. Studies on glycopeptides released by trypsin from intact human erythrocytes. Biochemistry. 1967 Jul;6(7):2195–2202. doi: 10.1021/bi00859a042. [DOI] [PubMed] [Google Scholar]


