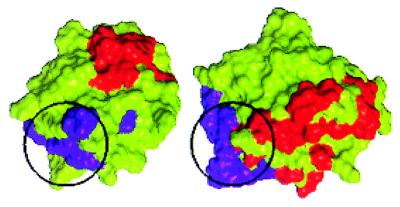
Figure 5.
Comparison of the spatial orientation of the primary (purple) and secondary (red) receptor-binding sites of FGF-2 and the primary (purple) and secondary (red) receptor-binding sites of IL-1β (31). The Cα traces of the FGF-2 molecule and the IL-1β molecule were superimposed. The FGF-2 molecule was then translated horizontally for comparison. The primary binding site (highlighted with a black circle) of FGF-2 forms a part of the primary binding site of IL-1β [identified on the basis of the interaction with receptor domains I and II in the cocrystal structure (31)]. The relative spatial orientations of the two primary binding sites are similar (when superimposed they are at the bottom left of the molecules). The number of residues constituting the IL-1β primary binding site is much higher than in FGF-2. Although the three-dimensional orientations of the set of noncontiguous amino acids that confer receptor binding are very similar in the two proteins, there is no one-to-one correspondence in the amino acid composition at the receptor-binding sites. Note also that the secondary binding sites (red), which are believed to provide functional specificity, are spatially located differently in FGF-2 and IL-1β.